Heartwarming Butane Reacts With Oxygen Balanced Equation

Give the complete and balanced chemical equation for the reaction between butane eqC_4H_10 eq and oxygen.
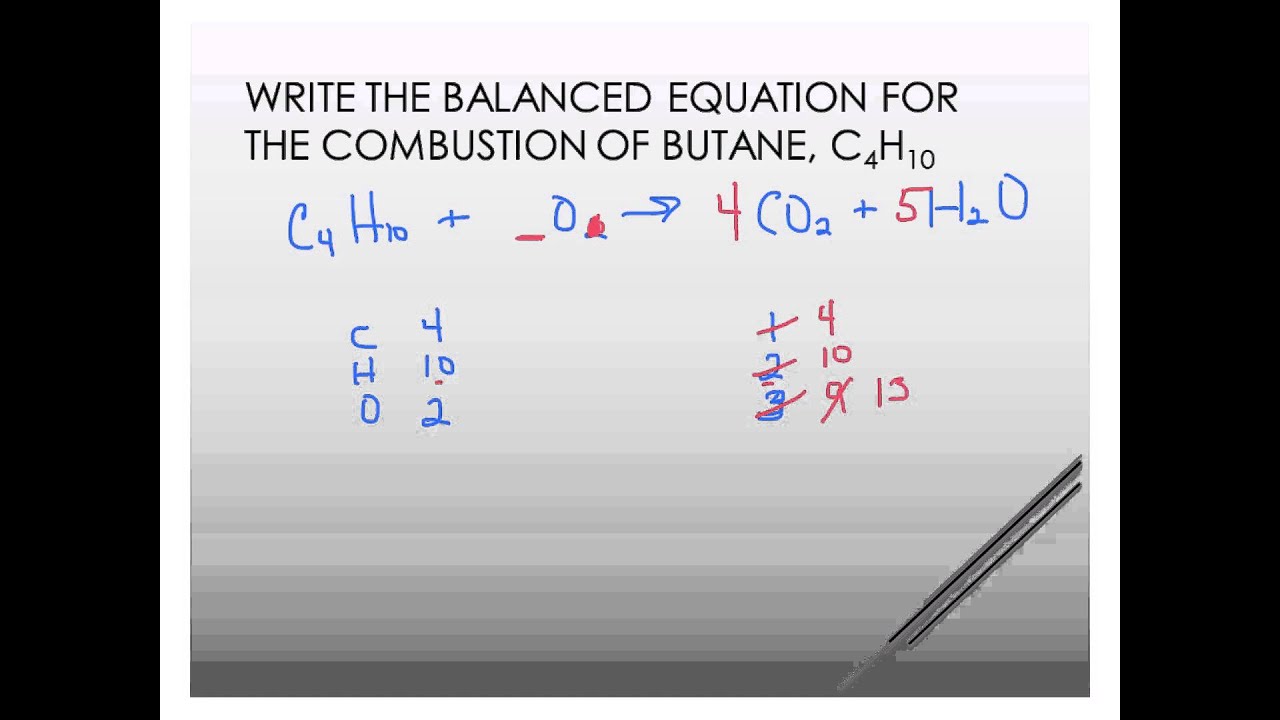
Butane reacts with oxygen balanced equation. Combustion of 1 mole of Butane we start with a balanced chemical equation. The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water. The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water.
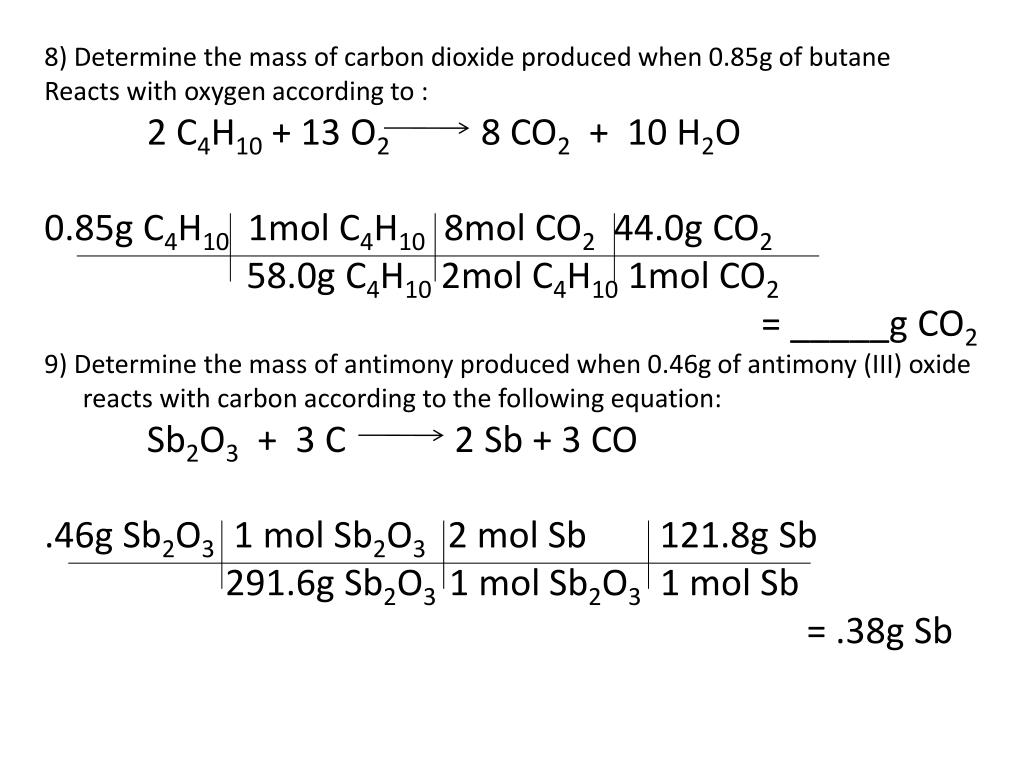
When butane C4H10 reacts with oxygen. Butane C4H10 is a common fuel used for heating homes in areas not served by natural gas. 8 mol CO 2.
We are asked to take the following word equation and turn it into a balanced chemical equation. Try to start with atom having higher number like u have 2 Oxygen atoms and 1 Carbon atom on LHS but only 1 C and 1 O on LHS. All parts of this question are related to this reaction.
Combustion of hydrocarbons to carbon dioxide and water requires that i we balance the carbons as carbon dioxide. Butane is a gas at room temperature and pressure. Butane is fed to an experimental combustion chamber at the rate of 100 grams per hour.
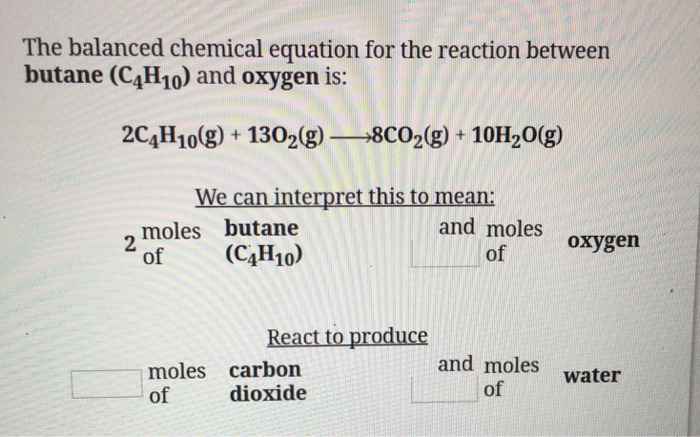
2C4H1013O28CO210H2O How many grams of water are produced when 2154 grams of butane react. The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water. Butane C4H10C4H10 reacts with oxygen O2O2 to form water H2OH2O and carbon dioxide CO2CO2 as shown in the following chemical equation.
The chemical formula of butane is C4H10. The equation for its combustion is 2C4H10 13O2 -- 8CO2 10H2O. - how many grams of butane can be burned by 254 moles of oxygen.