Looking Good Chemical Equation For Rust Formation
Since the chemical equation consists of symbols we can think of this as a symbolic representation.
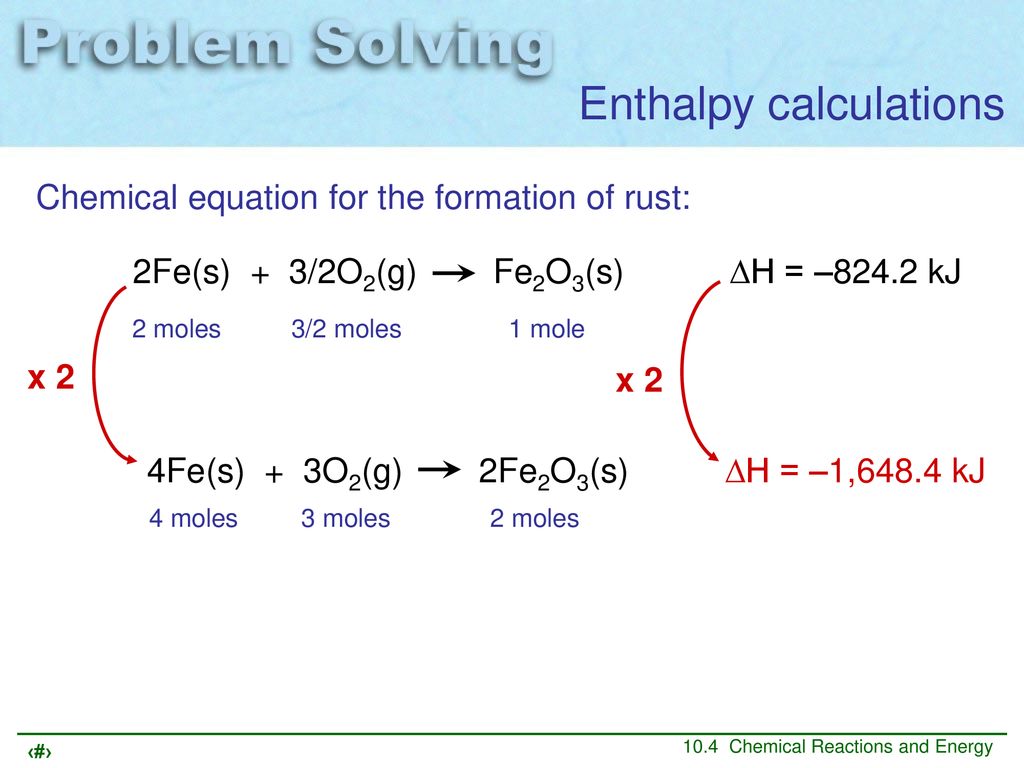
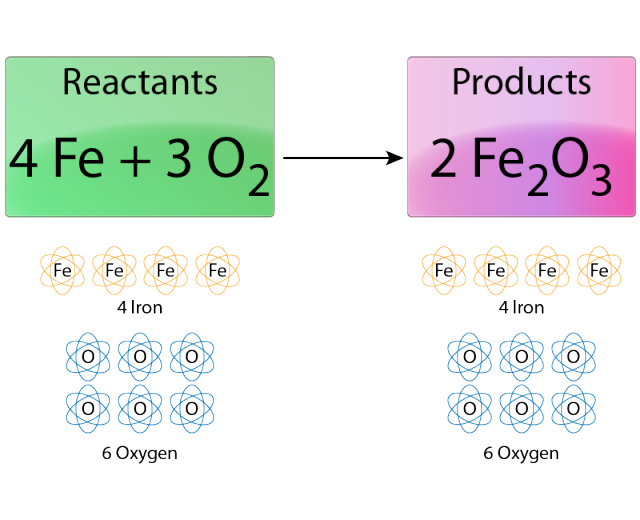
Chemical equation for rust formation. Complete info about it can be read here. Water is also required for this reaction to occur but because the total amount of water does not change it is not included in the equation. 4Fe 3O2 2Fe2O3.
The chemical formula for rust is Fe 2 O 3nH 2 O The overall chemical equation for the formation of rust is Iron water oxygen rust 4 Fe s 6 H 2 O l 3 O 2 g 4 Fe OH 3 s. 2Fes 2H 2 Ol O 2 g 2Fe 2 aq 4OH-aq. Although about 21 of air consists of oxygen 1 rusting doesnt occur in dry air.
The chemical equations for rust formation. Write a chemical equation for reaction of iron oxide with aluminium to form aluminium oxide and iron CBSE Class 10 CBSE Class 10 Science Write the balanced chemical equations for the following reactions and identify the type of reaction in each case. From the equation for rusting you can see that four atoms of iron combine with three molecules of oxygen to form two molecules of iron oxide.
Chemical equation for the formation of rust to know how to measure a reaction rate. Rust consists of hydrous iron III oxides Fe 2 O 3 nH 2 O and iron III oxide-hydroxide FeO OH Fe OH 3 and is. In this experiment youll discover what kind of conditions help rust form or prevent it.
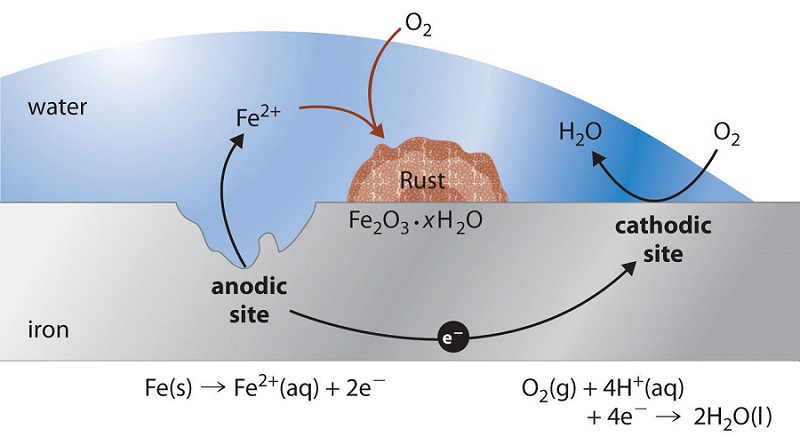
For H2O there is one atom of oxygen and two atoms of hydrogen. Thus iron rusts faster in contact with salt water than in fresh. Rust is an iron oxide a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture.
Rust is usually formed on the surface of iron when exposed to any air moisture or water. The reactants of this chemical reaction are iron water and oxygen and the product is hydrated iron oxide better known as rust. Several forms of rust are distinguishable visually and form under different circumstances.








:max_bytes(150000):strip_icc()/BalanceEquations1-56a132765f9b58b7d0bcf535.png)
