Neat Endothermic Word Equation
A Endothermic b Exothermic 7 Which of these is the odd one out.
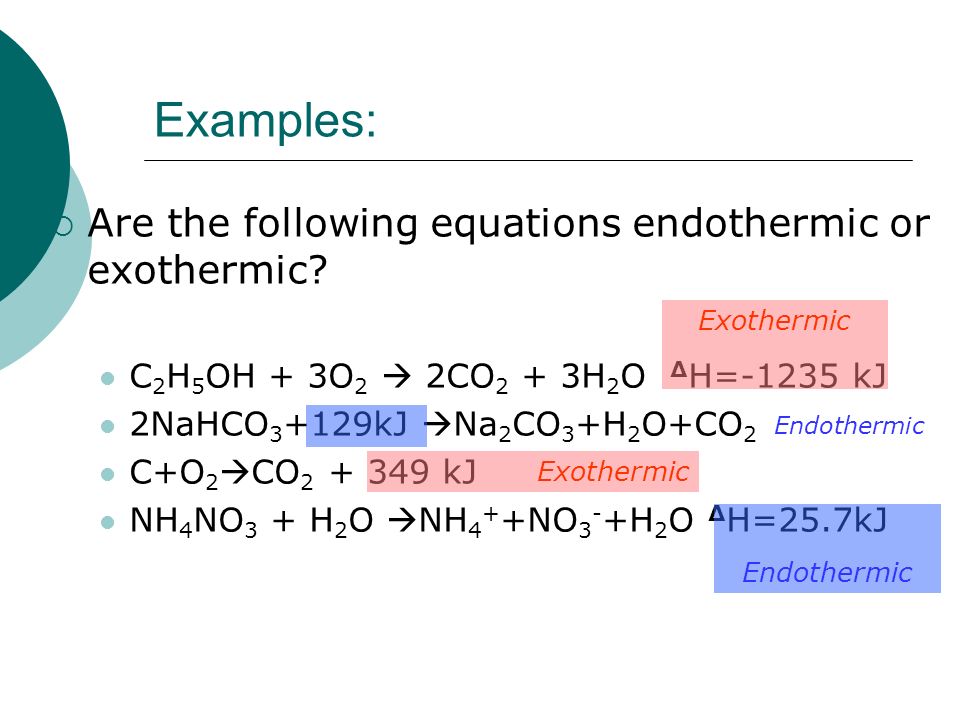
Endothermic word equation. You have to either measure the heating or cooling or know enough about similar types of reactions. In a chemical equation the word heat or the triange symbol are used to indicate if the reaction is exothermic or endothermic. You cant in general tell whether a reaction is exothermic or endothermic just by the form of the equation.
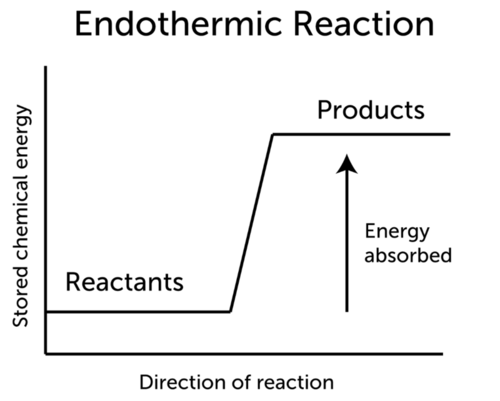
Calcium carbonate heat Calcium oxide carbon dioxide is the word equation for the endothermic reaction. An endothermic reaction uses energy as a reactant. An endothermic process is any process with an increase in the enthalpy H or internal energy U of the system.
Where does the energy come from in Endothermic Reactions. When a chemical reaction occurs energy is transferred to or from the surroundings. All combustion reactions are exothermic reactions.
Thus Na HOH -- NaOH heat would indicate the reaction is. Only at temperatures T where TΔRSΔRH an endothermic reaction may become exergonic. In such a process a closed system usually absorbs thermal energy from its surroundings which is heat transfer into the system.
They absorb heat energy from their surroundings. A Methane burning in air b Neutralisation c Iron oxidising d Thermal decomposition e Magnesium and hydrochloric acid 8 What word means the chemicals that are used up in the reaction. The main difference is endothermic absorbs heat while exothermic produces heat.
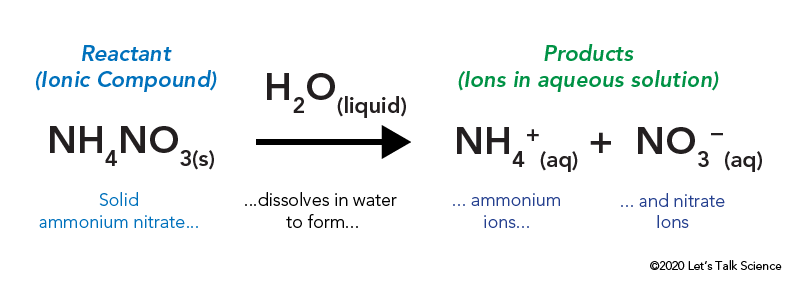
NH4Cl s H2O l NH4Cl aq Heat. There is usually a temperature change. Endothermic reactions are the opposite of exothermic reactions.