Beautiful Work H2 O2 H2o Exothermic Endothermic
A negative delta H means the heat is given up by the reaction.
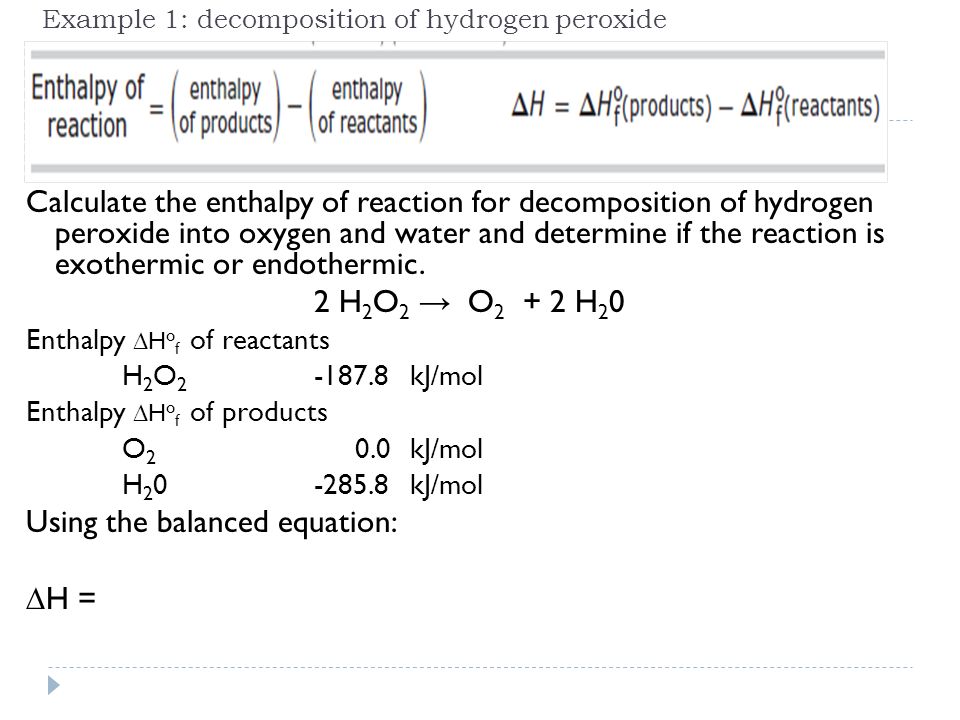
H2 o2 h2o exothermic endothermic. An equation for the reaction is. Add approximately 50 ml of water to a large test tube. Delta H3 Using Hesss law the sum of the deltas is.
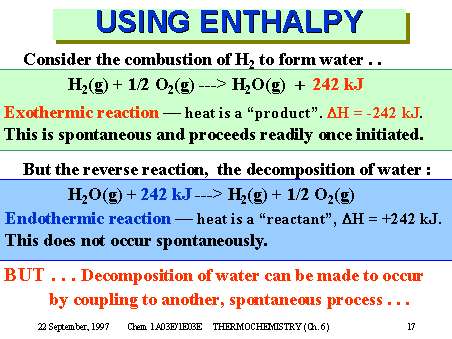
Answer to The conversion of H2O to H2 and O2 is an endothermic reaction. Delta H2 H2Og --yields-- H2Os. Is this reaction endothermic or exothermic.
B Caculate rxnH if 600 moles of hydrogen gas are reacted in an abundance of oxgyen. Consider the following reaction. A delta H means the heat is added to the reaction.
A condensation polymerization reaction produces a polymer and 1 H2 2 O2 3 CO2 4 H2O Which statement best describes the reaction pathway graph for an exothermic reaction but not an end. Gently stir with the thermometer until all calcium chloride has been dissolved. An in Chemistry if youre in doubt about the correctness of the answers or theres no answer then try to use the smart search and find answers to the similar questions.
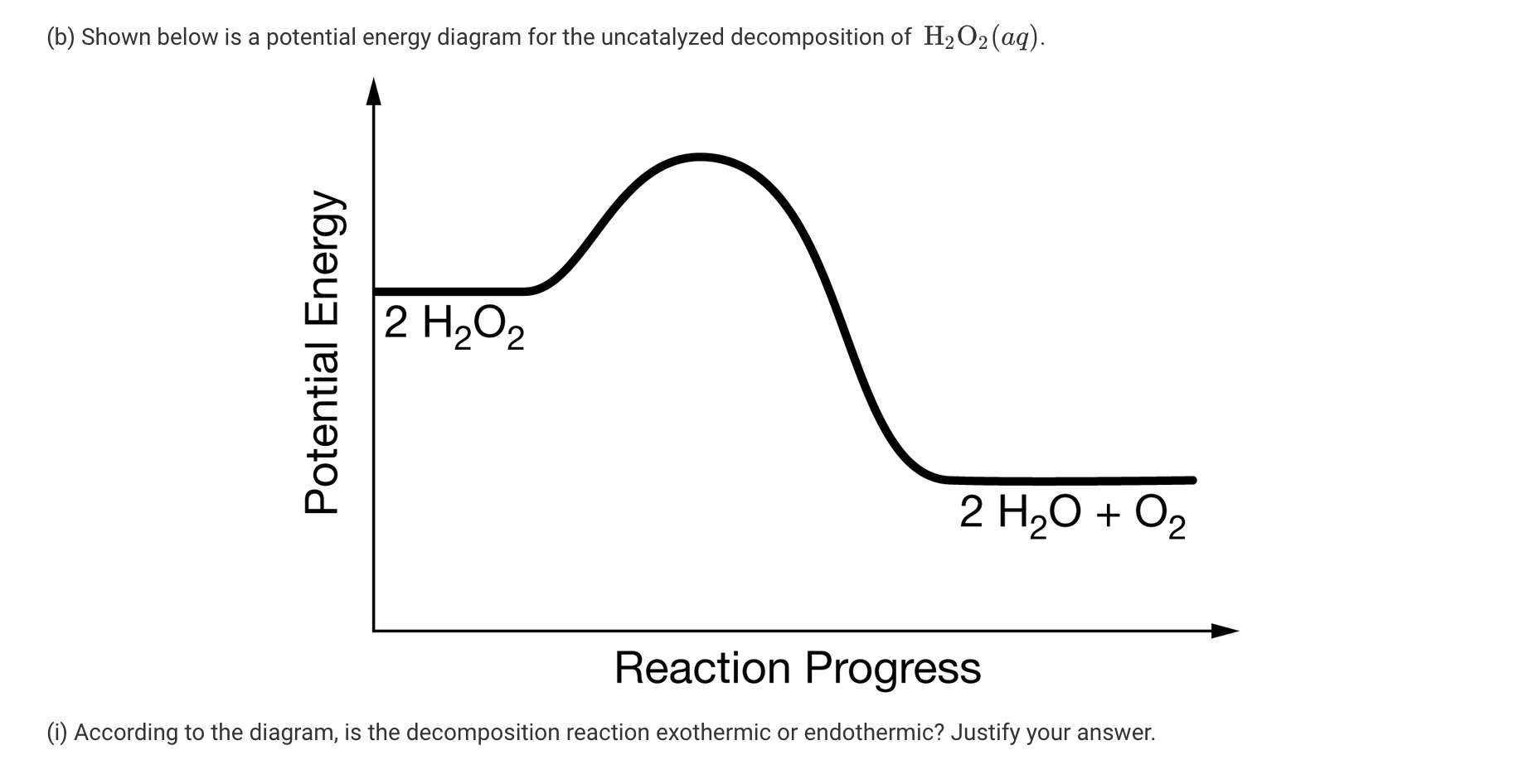
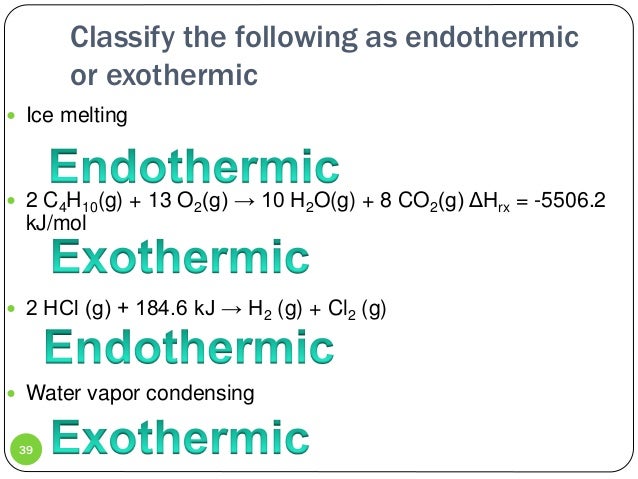
The reaction scheme as written does not occur. Describe what is happing to the energy during this reaction. 2 H2g O2g 2 H2Og ΔH -484 kJ Is the reaction exothermic or endothermic.
2 Al 32 O2 H 1676 kJ Select endothermic exothermic. H2g ½ o2g h2og δh -5782 kcal ½n2g o2g 81 kcal no2g ½n2g 32h2g nh3g 110 kcal. Record the temperature of the water.