Outrageous Incomplete Combustion Of Carbon Equation
Methane oxygen carbon dioxide and water.
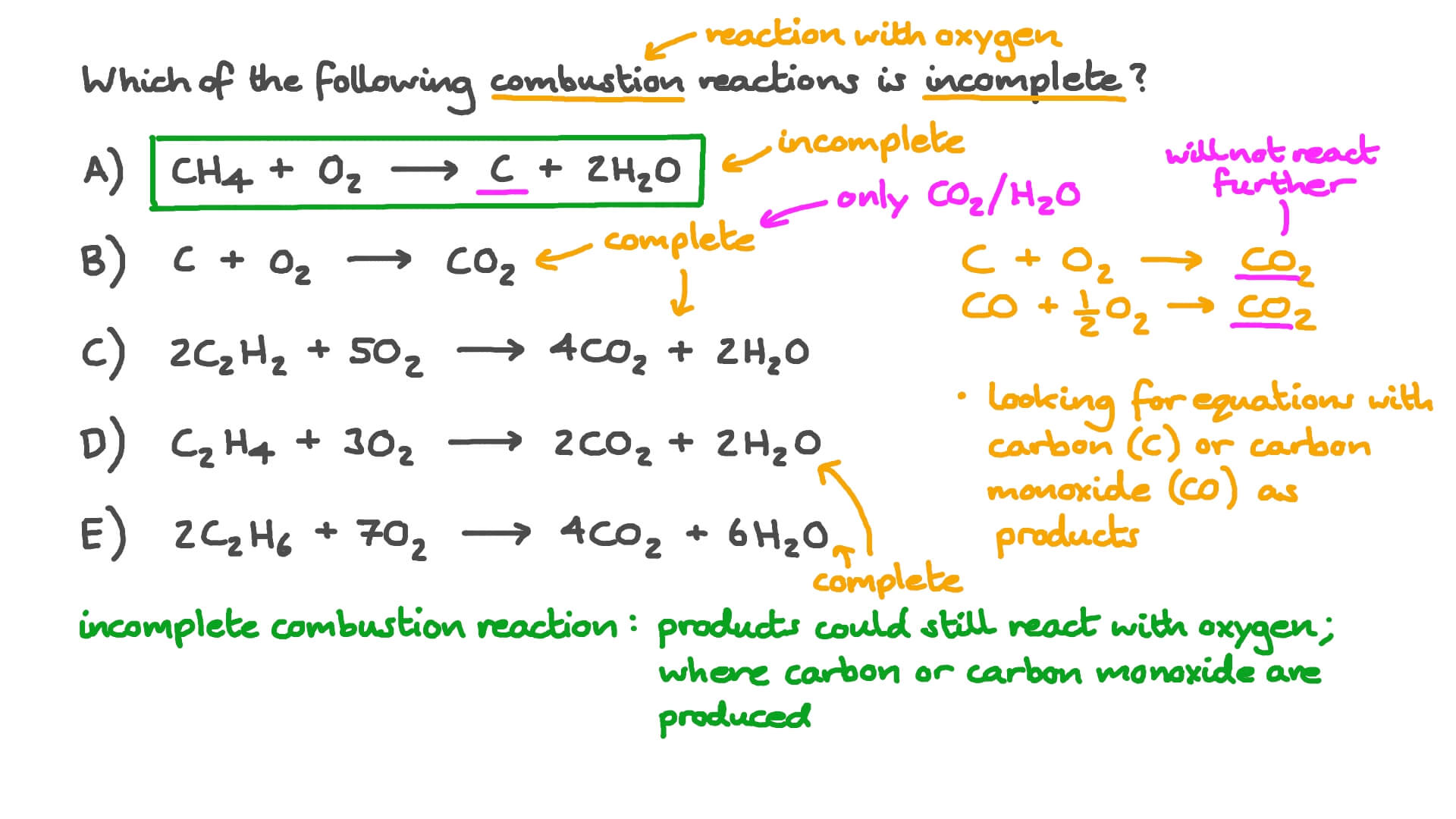
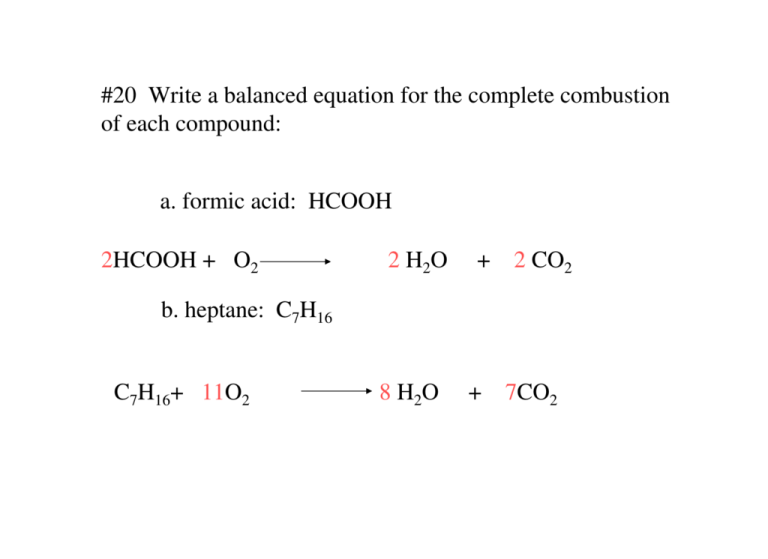
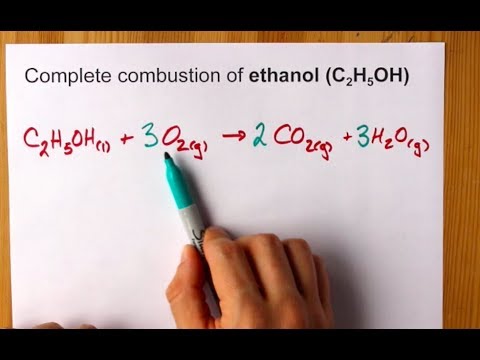
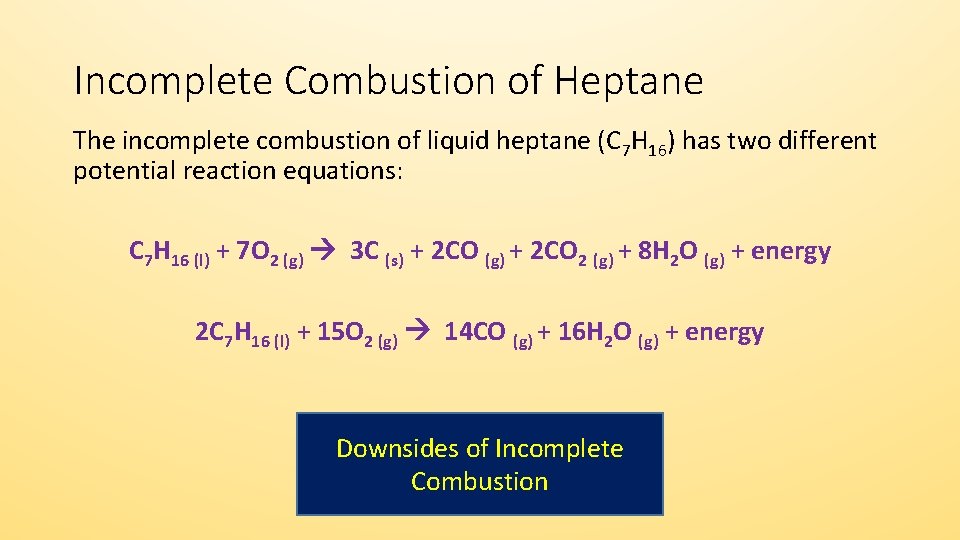
Incomplete combustion of carbon equation. C 10 H 20 27 2 O 2 8 C O 2 g C O g C s 10 H 2 O l Δ Is this balanced with respect to mass and charge. As a simple way of thinking about it the hydrogen in the hydrocarbon gets the first chance at the oxygen and the carbon gets whatever is. Incomplete combustion means burning in a lack of air not enough oxygenIf there is not enough oxygen available for all the carbon to turn into carbon dioxide see complete combustion then some or all of the carbon turns to carbon monoxideThis happens with.
Traditionally on large multiburner plants getting the fuel and air. Incomplete combustion uses fuel inefficiently and the carbon monoxide produced is a health hazard. Click to see full answer.
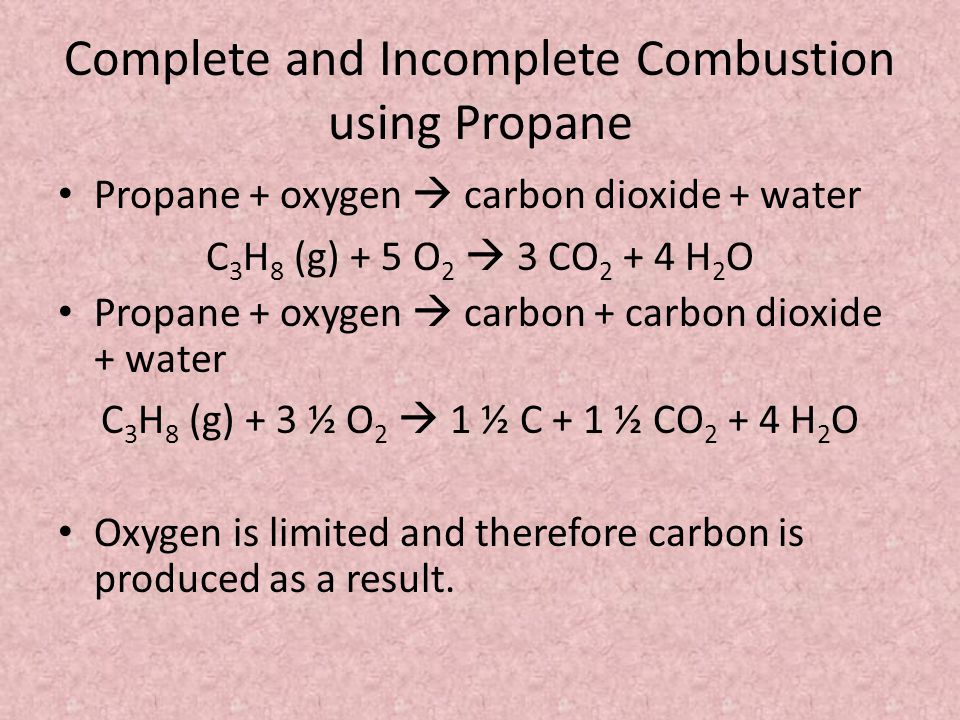
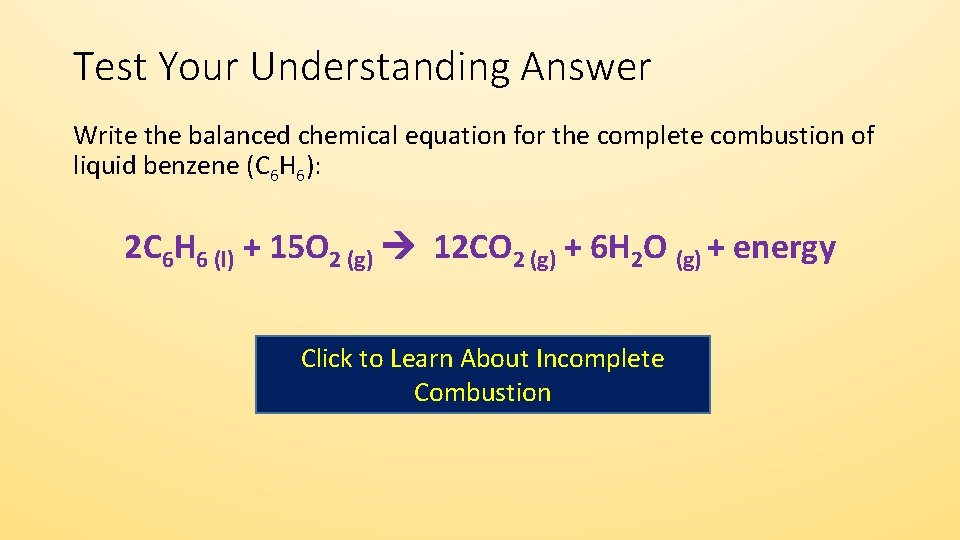
Hydrocarbon oxygen carbon monoxide carbon water The carbon is released as soot. 2 C6H6 l 15 O2 g -- 12 CO2 g 6 H2O g Calculate the percent yield of the reaction if the combustion of 136 g C6H6 MW 7812 gmol with excess O2 MW 3200 gmol produced 401 g CO2 MW 4401 gmol 5 years ago. Incomplete combustion occurs when a combustion reaction occurs without a sufficient supply of oxygen.
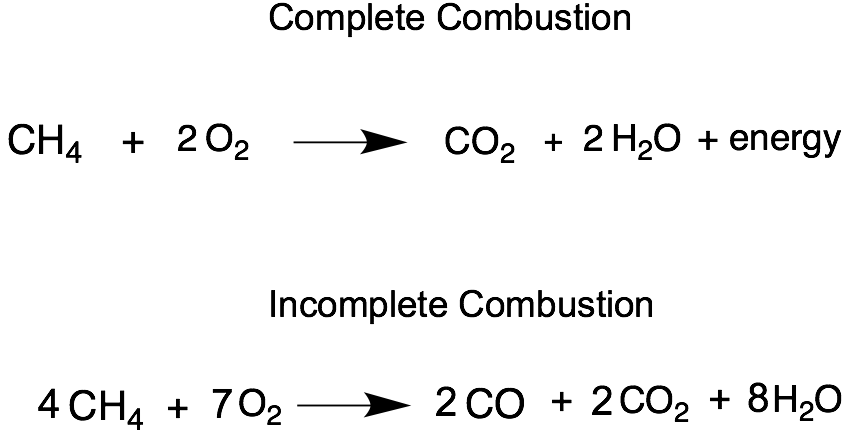
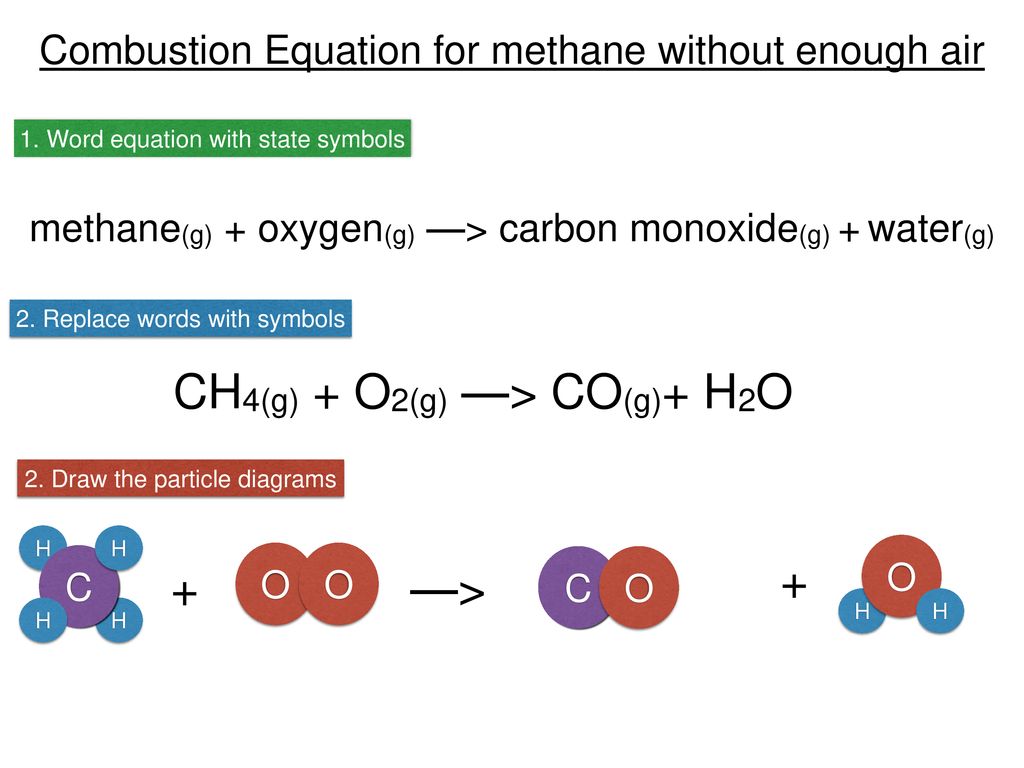
Incomplete combustion where there is not enough oxygen present can lead to the formation of carbon or carbon monoxide. During incomplete combustion part of the carbon is not completely oxidized producing soot or carbon monoxide CO. Carbon monoxide is a.
If the ratio of carbon dioxide to carbon monoxide formed is 91 when x cm3 of ethene is burnt what is the volume of oxygen gas consumed in the reaction. The Combustion of Hydrocarbons - Chemistry. Operationally this is used to optimize the combustion process as it indicates that there is a nonoptimum mixing of combustion air with the fuel.
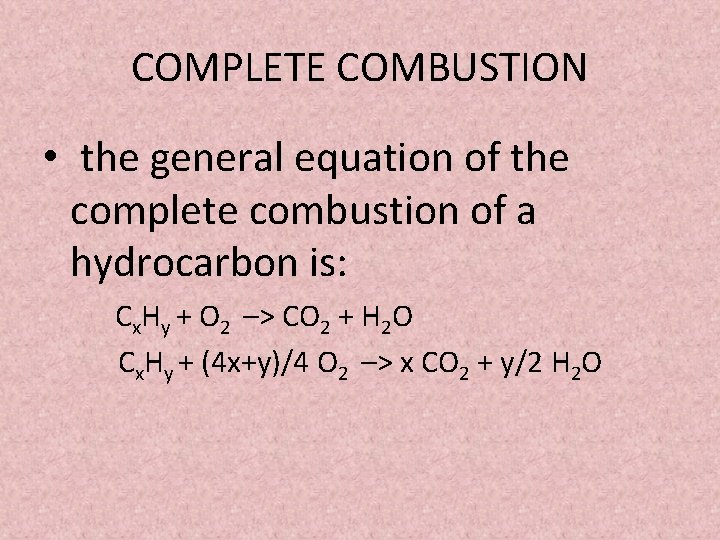
The complete combustion of hydrocarbons leads to carbon dioxide and water formation while incomplete combustion yields carbon dioxide carbon monoxide soot and water. Water is still produced but carbon monoxide and carbon are produced instead of carbon dioxide. The incomplete combustion of fossil fuels results in the formation of carbon monoxide and soot carbon.