Recommendation Methane Plus Oxygen Balanced Equation
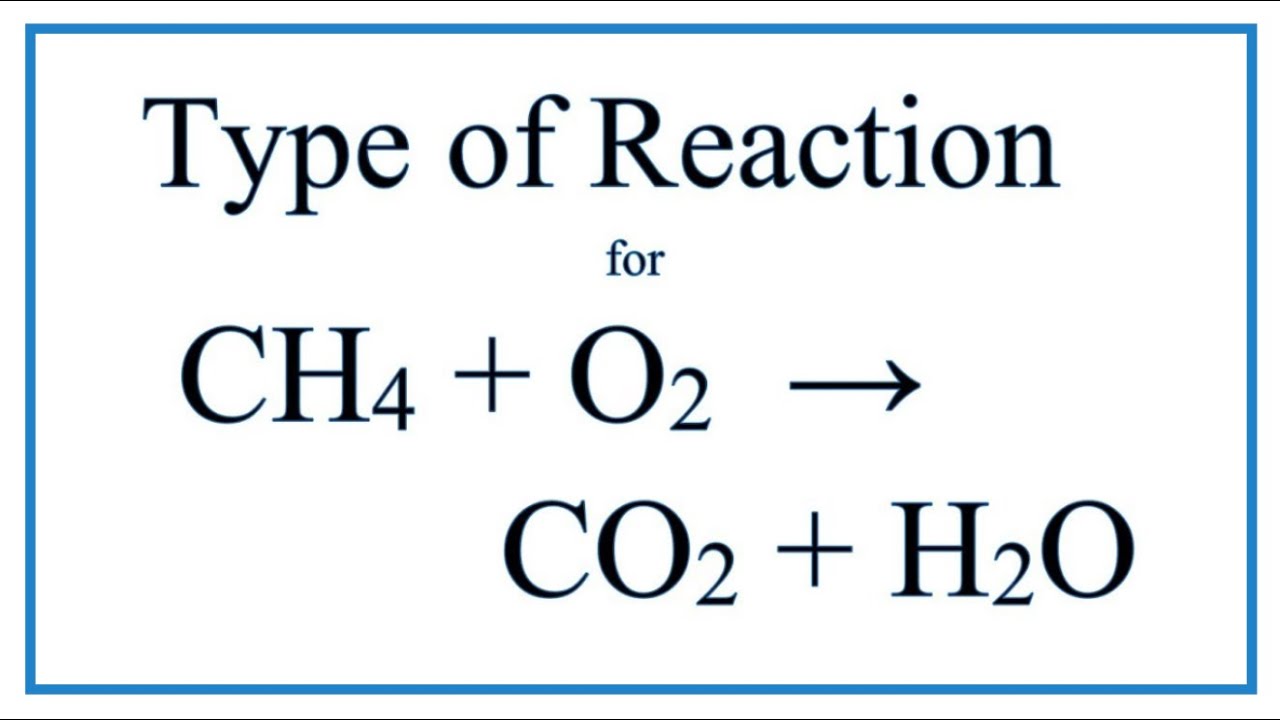
CH 4 2O2 CO2 2H 2O This is the balanced reaction equation for the combustion of methane.
Methane plus oxygen balanced equation. Learners use the word equation to chunk the symbol equation into smaller pieces. The Reaction between methane gas and oxygen gas in the atmosphere 2CH4 4O2 2CO2 4H2O The Reaction between methane gas and oxygen gas in the atmosphere Balanced equation for methane plus oxygen to form carbon dioxide plus water. The balanced equation for the incomplete combustion of methane is.
CO₂CO Balanced EquationCarbon plus Oxygen yields Carbon monoxide Balanced EquationRELATED SEARCHEScarbon reacts with oxygen to form carbon dioxide balanc. The reaction between methane with oxygen is an example of combination reaction. 2 CH4 g 3 O2 g 2 CO g 4 H2O g Like all hydrocarbons the combustion of methane forms water due to the presence of hydrogen but produces carbon dioxide as a result of complete combustion and carbon monoxide dangerous as a result of incomplete combustion.
In the reaction the bonds in the methane and oxygen come apart the atoms rearrange and then re-bond to form water and carbon dioxide. What is the molar ratio of methane to oxygen. The equation is balanced because the number of atoms for every element is the same on both the reactant and the product sides.
With the proper coefficients the reaction equation shows that the same number of. One mole or 1 molecule or 16 g of C H 4 reacts with 2 moles or 2 molecules or 64 g of oxygen to give one mole or 1 molecule or 44 g of C O2. All the atoms in the reactants form the products so the mass of the reactants and the products is the same.
Methane CH4 C H 4 ammonia N H3 N H 3 and oxygen O2 O 2 can react to form hydrogen cyanide HCN H C N and water according to this equation. The balance equation is given by CH4 2O2 CO2 2H2O The reaction between methane with oxygen is. In this video well balance the equation CH4 O2 CO2 H2O and provide the correct coefficients for each compoundIn order to balance CH4 O2 CO2 H.
Methane burns with oxygen to form carbon dioxide and water. CH4 plus 2O2 reacts to form CO2 plus 2H2O. CH4N H3O2 HCN H2O C.