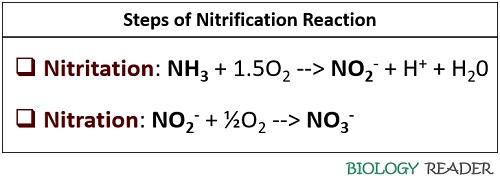
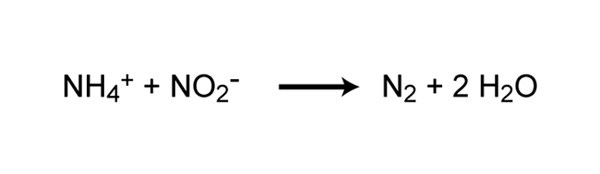
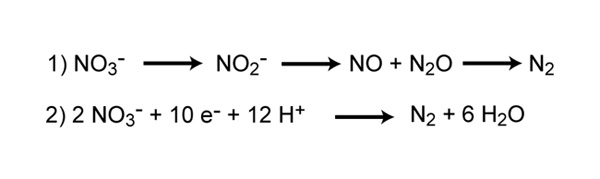
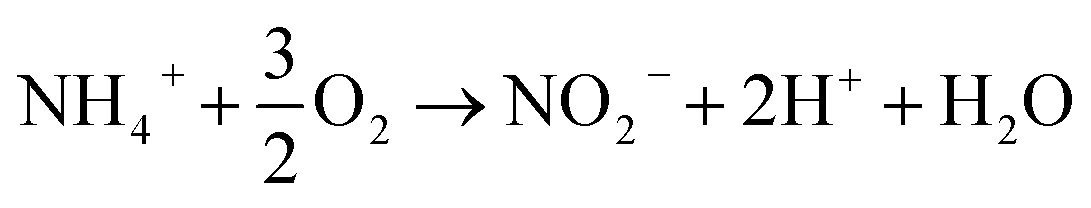Neat Nitrification Chemical Equation
During nitritation nitrosomonas convert NH3 ammonia into NO2 nitrogen dioxide.
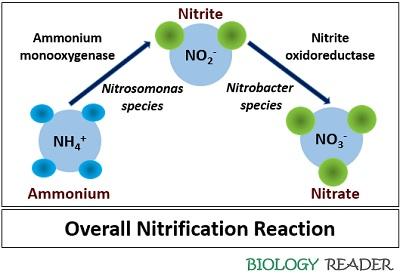
Nitrification chemical equation. Basic Nitrification Facts Nitrification is the biological removal oxidation of ammonia NH4 by certain bacteria in the presence of oxygen. Nitrate is a nitrogen oxoanion formed by loss of a proton from nitric acid. Two species of bacteria are involved in the process Nitrosomonas and Nitrobacter.
It is a conjugate base of a nitric acid. 1979 with modifications for soil temperature moisture and pH Williams et al 2008a 50. Volatilization of NH 3 is calculated concurrently with nitrification but N 2 O production is not associated with it.
Nitrosomonas carry out the first step of the process producing nitrite. Denitrification is an anaerobic process that is carried out by denitrifying bacteria which convert nitrate to dinitrogen. The nitrification process is carried out by two different types of bacteria.
Define Biological Nitrification yNitrosomonas convert ammonia to nitrite. Nitrite and nitrate are produced during nitrification through ammonia utilization by nitrifying bacteria. Nitrification is an aerobic microbial process by which specialized bacteria oxidize ammonium to nitrite and then to nitrate.
Is a liquid which is readily ignited when in. The first stage is the oxidation of ammonium NH 4. The chemical reaction between silverI nitrate and magnesium chloride results in the formation of silverI chloride and magnesium nitrate.
These bacteria are collectively known as nitrifiers and are autotrophic ie. Classical Equation For The Nitrification Process NH4 O 2 Nitrosomonas sp. Principal species present at pH 73.