Fine Beautiful Phosphoric Acid Reacts With Magnesium Hydroxide

May react with active metals including such structural metals as aluminum and iron to release hydrogen a flammable.
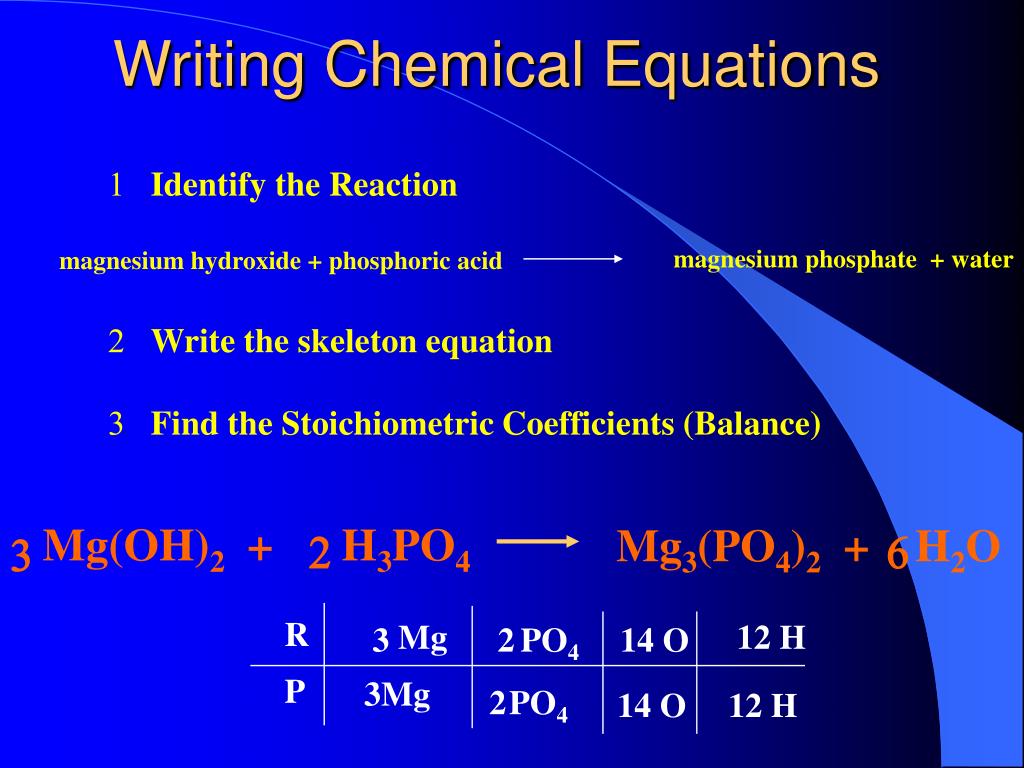
Phosphoric acid reacts with magnesium hydroxide. PHOSPHORIC ACID reacts exothermically with bases. Phosphoric acid reacts with magnesium hydroxide to produce magnesium phosphate and water via the following reaction. What is the limiting reactant.
Magnesium react with phosphoric acid 3Mg 2H 3 PO 4 Mg 3 PO 4 2 3H 2 Check the balance Magnesium react with phosphoric acid to produce magnesium orthophosphate and hydrogen. What is the balanced equation for magnesium hydroxide. Magnesium hydroxide react with phosphoric acid 3Mg OH 2 2H 3 PO 4 Mg 3 PO 4 2 6H 2 O Check the balance Magnesium hydroxide react with phosphoric acid to produce magnesium orthophosphate and water.
Phosphoric acid or phosphates ionize in solution to form H2PO4 HPO42 andor PO43 and these anions adsorb onto Mg OH H2Ox to inhibit the formation of Mg OH2 and further promote the. In a neutralization reaction 5000 mL of 08190 M phosphoric acid reacts with 2485 g of magnesium hydroxide producing magnesium phosphate and water. Phosphoric acid reacts with magnesium hydroxide to produce magnesium phosphate and water via the.
What is the hink-pink for blue green moray. Phosphoric acid and magnesium hydroxide react in a neutralization reaction to produce magnesium phosphate and water. Write a balanced equation for the reaction.
In a neutralization reaction 5000 mL of 08500 M phosphoric acid reacts with 2500 g of magnesium hydroxide producing magnesium phosphate and water. How much of the excess reactant remains after the. Magnesium sulfide reacts with hydrochloric acid to produce magnesium chloride and hydrogen sulfide.
What type of reaction is magnesium sulfide. Phosphoric acid reacts with magnesium hydroxide to produce magnesium phosphate and water via the following reaction. Write a balanced equation for the reaction.