Great Sulfuric Acid + Ammonia Balanced Equation

As a result of the reaction of potassium permanganate KMnO 4 sulfuric acid H 2 SO 4 and ammonia.
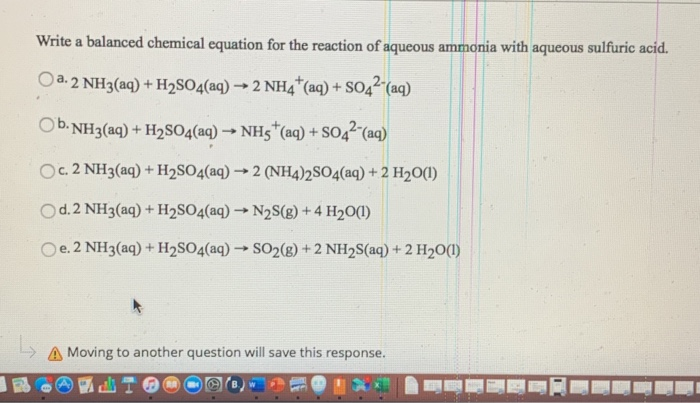
Sulfuric acid + ammonia balanced equation. Write word equations and balanced equations for the following combination reactions. Ammonia and sulfuric acid react to form ammonium sulfate. Aqueous ammonia is ammonia in water.
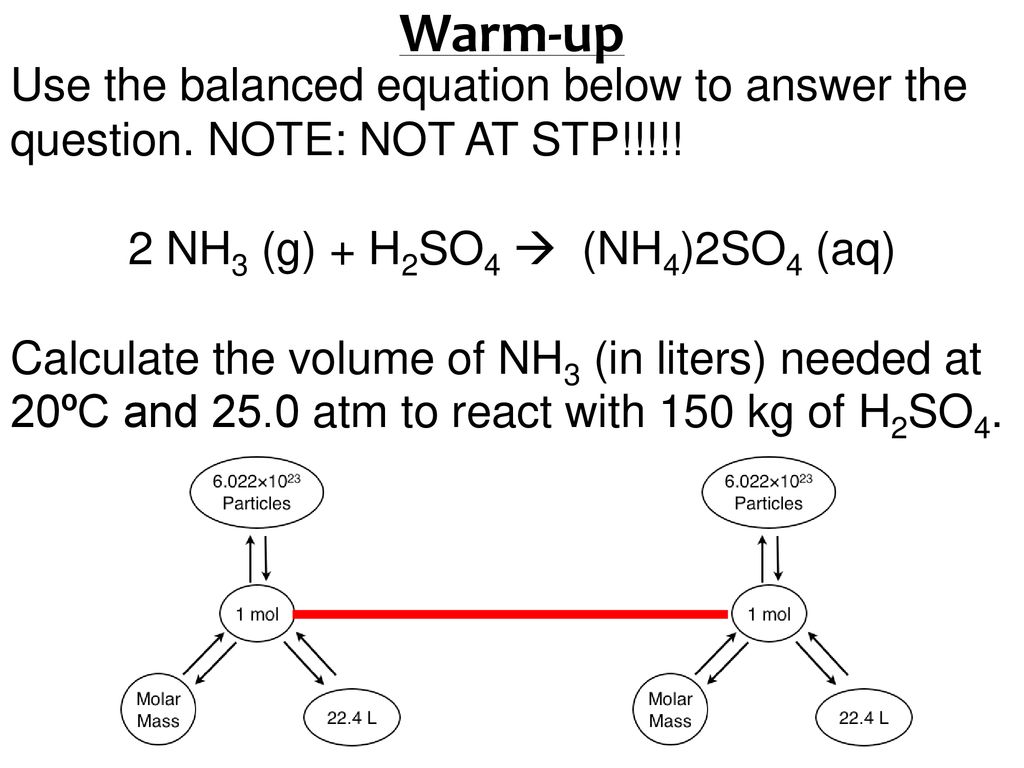
What Volume Of 0250M Sulfuric Acid Solution Would Be Needed To React Complately With 1800 ML Of 0350M Ammonia Solution. HBr aq NH 3aq--- To write the products we combine the anion of the acid with the cation of the base and write the correct formula following the principle of electroneutrality. What volume of 0250M sulfuric acid solution would be needed to react complately with 1800 mL of 0350M ammonia solution.
The other product is water. Consider the reaction between hydrobromic acid and ammonia. A seething mass of bubbles form as this carbon dioxide escapes from the solution.
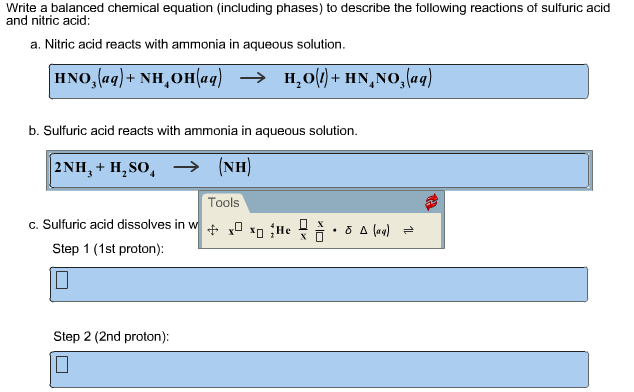
When ammonia is added to water it reacts as follows. This is an interesting reaction since it produces H2SO4 sulfuric acid. Dissolved ammonia reacts with sulfuric acid to form dissolved ammonium sulphate and water.
For this reaction we have a combination also called synthesis reaction. Ammonium sulfate is made by treating ammonia with sulfuric acid. The balanced equation for reaction between ammonia and sulfuric acid is.
What happens when ammonia reacts with Sulphuric acidWhat is the chemical formula for ammonium sulphate. SO 2 O 2 H 2 O H 2 SO 4. Be sure to count all of the.