Beautiful Work Unbalanced Chemical Equation Examples

Both of these sides are separated by the means of an arrow.
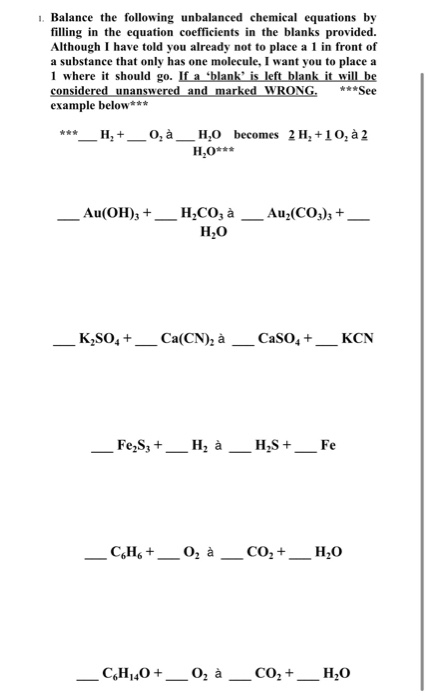
Unbalanced chemical equation examples. Balancing chemical equations is a basic skill in chemistry and testing yourself helps retain important information. A chemical equation is a symbolic representation of a chemical reaction in which the reactants and products are denoted by their respective chemical formulae. Try to start with the most complicated-looking group.
The word equations for a few of these reactions have been provided though most likely youll be asked to provide only the standard chemical equations. It is based on the law of conservation of mass Daltons atomic theory. Now back to balancing the example equation.
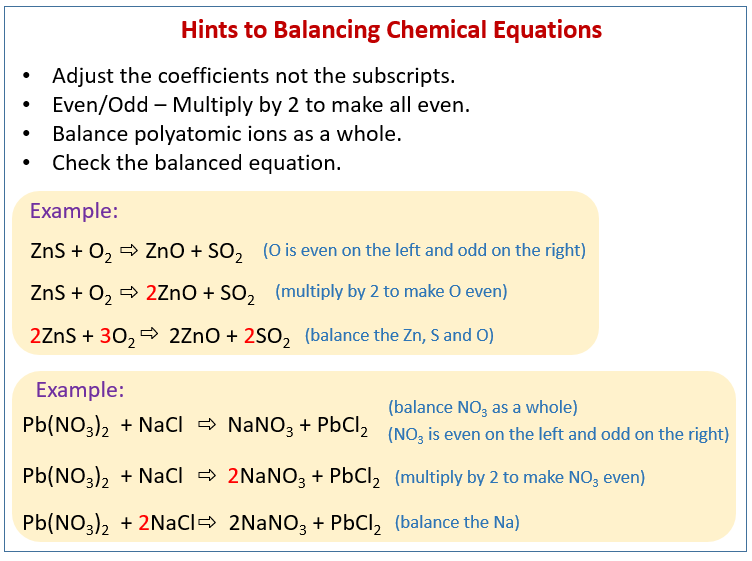
The unbalanced chemical equation must be obtained by writing the chemical formulae of the reactants and the products. Examples of using the twos and threes method to balance chemical equations even odd technique examples and step by step solutions Chemistry Balancing chemical equations Atoms can neither be destroyed nor created during a simple chemical reaction. We put a two in front of the water and this balances the oxygen.
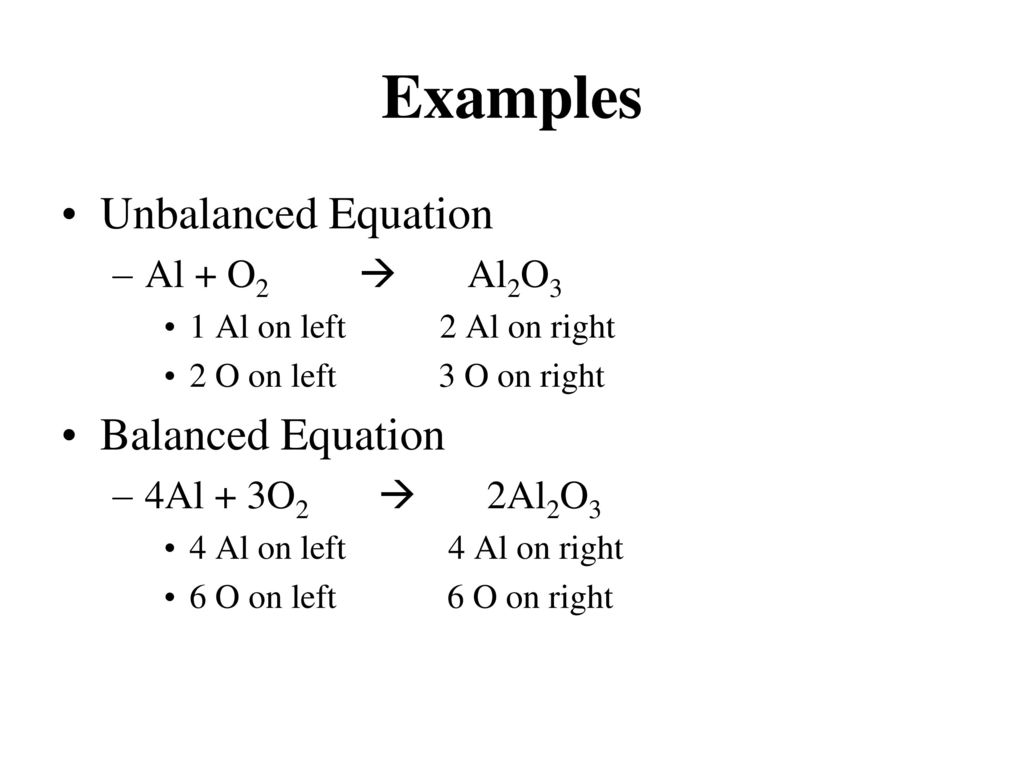
Parts of a Balanced Chemical Equation. A balanced chemical equation occurs when the number of the different atoms of elements in the reactants side is equal to that of the products side. This article is cited by 7 publications.
This process is represented qualitatively by an unbalanced chemical equation. Balancing chemical equations containing polyatomic ions Case 1. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button.
The balanced equation will appear above. Balanced or easy-to-balance chemical equations will be seen in the following examples. Ionic charges are not yet supported and will be ignored.