Formidable Balancing Chemical Equations Examples

All must have in common that the number of atoms for each of the elements will be the same before and after the arrow once the chemical equation has been balanced.
Balancing chemical equations examples. A Fe Cl2 FeCl3 b Fe O2 Fe2O3 c FeBr3 H2SO4 Fe2SO43 HBr d C4H6O3 H2O C2H4O2 e C2H4 O2 CO2 H2O f C4H10O O2 CO2 H2O g C7H16 O2 CO2 H2O h H2SiCl2 H2O H8Si4O4 HCl i HSiCl3 H2O. The reactant side and the product side. 2NaHCO 3 Na 2 CO 3 CO 2 H 2 O 32.
2Ag 2 O 4Ag O 2 28. The chemical equation has the products on the right side while the reactants are written on the left side. How to balance chemical equations.
We can only change the coefficients the number. 4K 2O 2 2K 2 O 29. This collection of ten chemistry test questions will give you practice in how to balance chemical reactions.
2Mg Cl 2 MgCl 2 27. Every balanced chemical equation consists of two parts. Parts of a Balanced Chemical Equation.
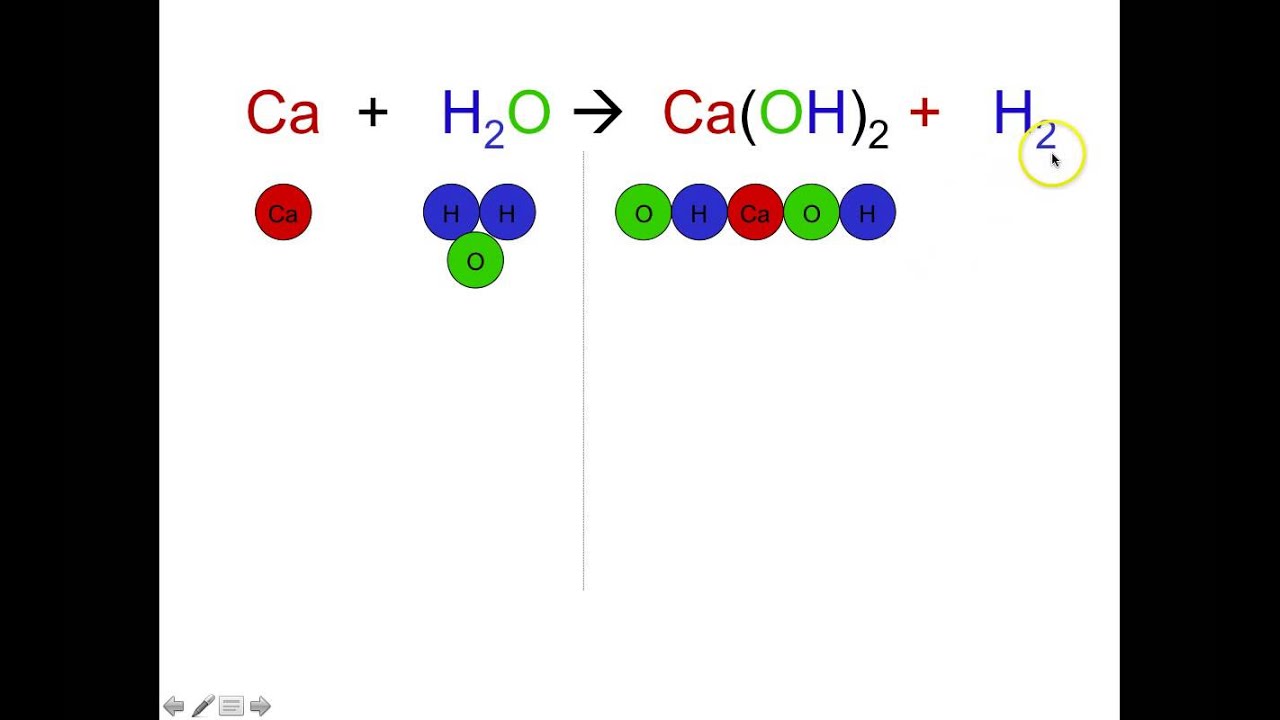
C5H12 O2--- 5CO2 H2O. The total number of atoms of an element present in a species in a balanced chemical equation is equal to the product of the stoichiometric coefficient and the number of atoms of the element in one molecule of the species. The following figure gives some hints on how to balance chemical equations.
Both of these sides are separated by the means of an arrow. For example O2 has 2 atoms of oxygen. 1 Balance the following chemical equation by writing coefficients in the blanks as necessary.