Impressive Define Balanced Chemical Equation

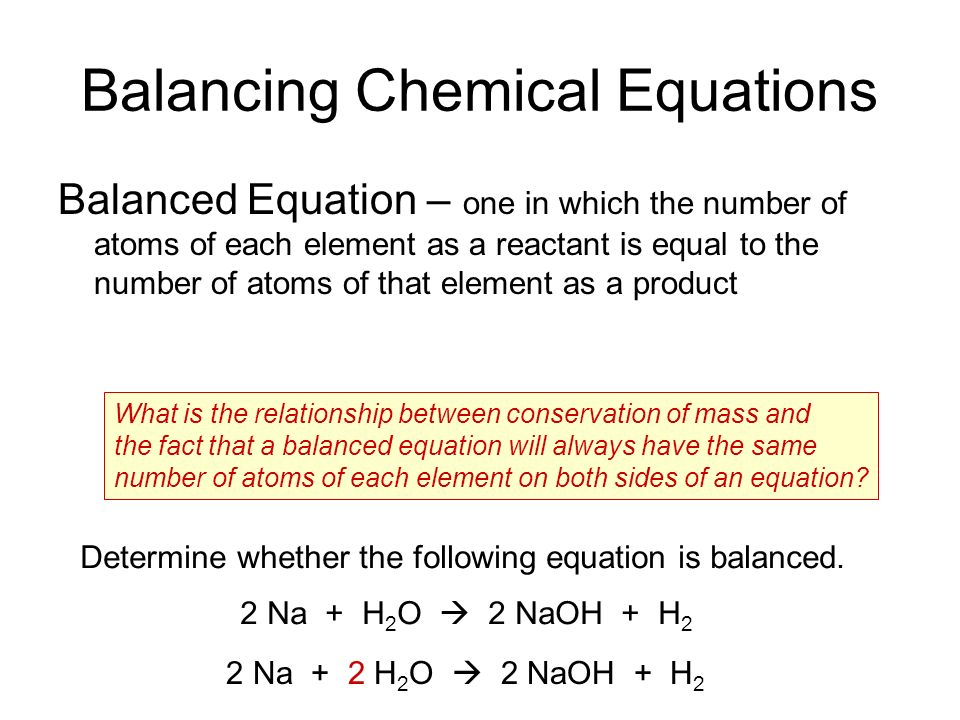
A balanced chemical reaction is an equation that has equal numbers of each type of atom on both sides of the arrow.
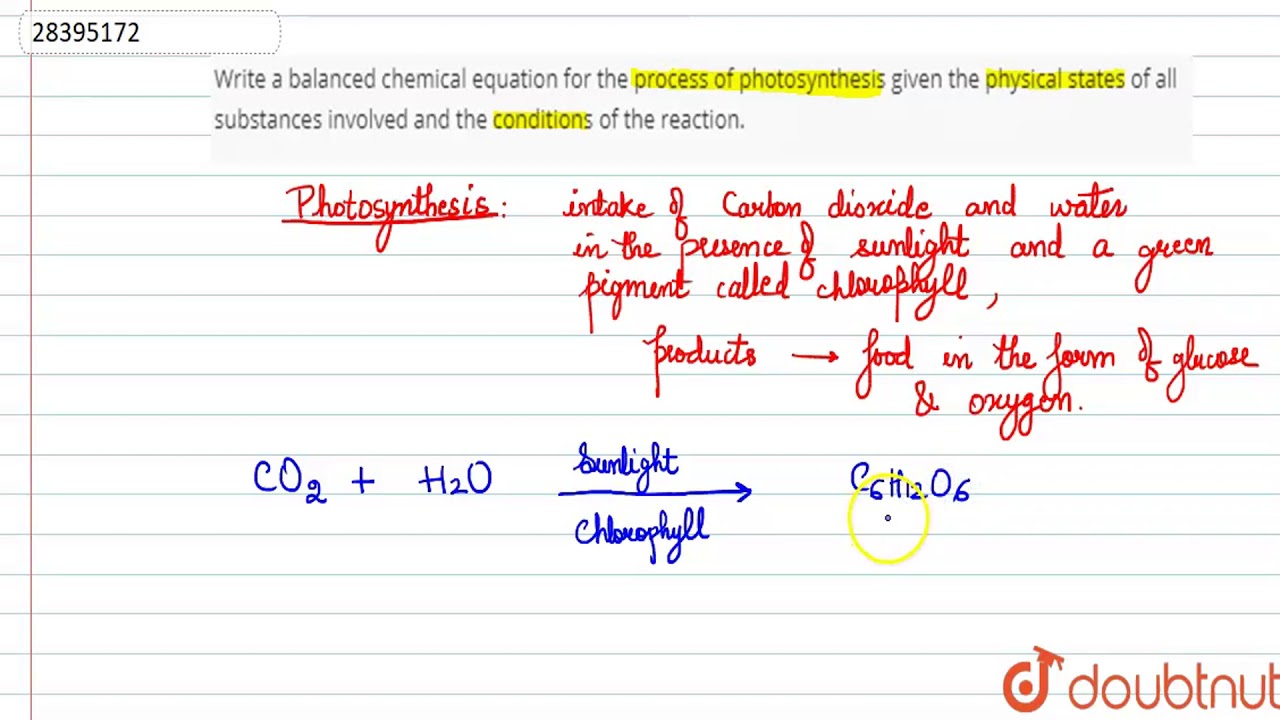
Define balanced chemical equation. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products. I Phosphorus burns in presence of chlorine to form phosphorus pentachloride. Another technique involves solving a system of linear equations.
B i P4 s 10Cl2 g 4PCl5 S iCH4 g 2O2 g CO2 g 2H2O l heat energy. Substances that react to these chemical changes are called reagents or reactants. A Balanced chemical equation has an equal number of atoms of different elements in the reactants and products.
Iii The process of respiration. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. Simple chemical equations can be balanced by inspection that is by trial and error.
According to law of conservation of mass matter can neither be created nor be destroyed in a chemical reaction. Balancing chemical equations for any chemical changes are very important matter. Ii Burning of natural gas.
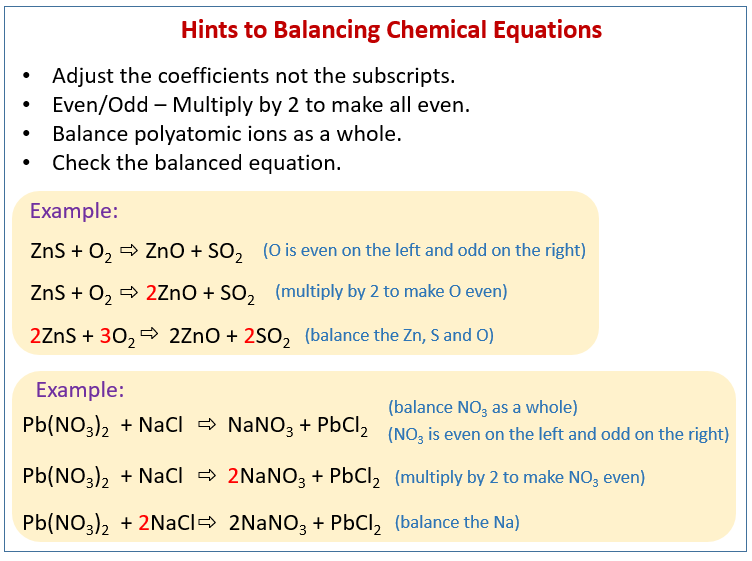
Now there are 4 molecules of hydrogen on products side so hydrogen is multiplied with 2 in the reactants side in order to balance the reaction. As there are 2 oxygen molecules on the reactants side 2 is multiplied on products side in order to balance the reaction. Fe Au Co Br C O N F.
A chemical equation that has an equal amount of each element on the left and right sides 2. In other words the mass and the charge are balanced on both sides of the reaction. Balanced chemical equation.