Beautiful Describe An Experiment To Show The Conditions Necessary For Rusting Of Iron

They rust faster in salty water or acid rain.
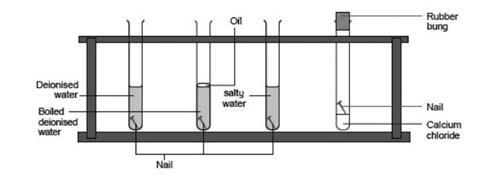
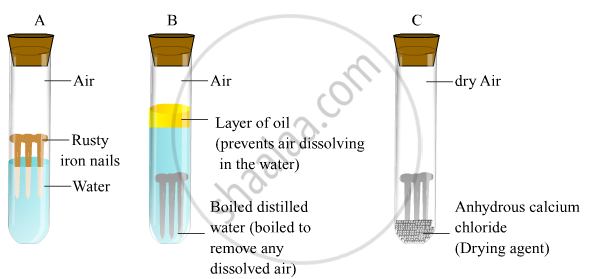
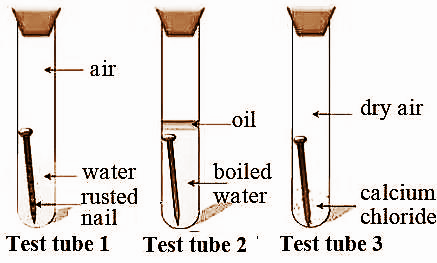
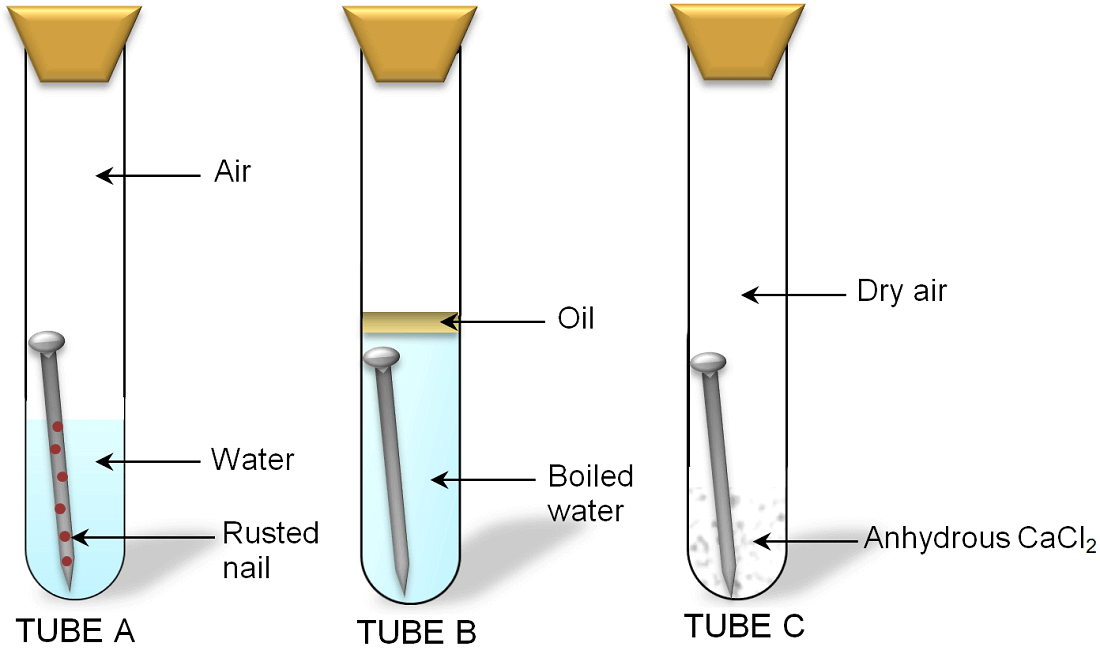
Describe an experiment to show the conditions necessary for rusting of iron. Rusting of iron is bad for economy. We take three test tubes and put clean iron nail in each of the them. Take three clean test tubes and mark them as X Y Z.
Then take three clean rust-free nails. Rusting is the oxidation of metal whereby the oxygen in the environment combines with the metal to form a new compound called a metal oxide. Put an iron nail in one test.
The final product of iron oxidation rust is usually a ferric oxide often hematite Fe 2 O 3. Conditions necessary for rusting experimenthttpsamznto3jFEVIdhttpsamznto3jBnisOThis Institute is for Science and Technology. Rusting takes place showing that air and moisture are necessary for rusting.
Answer verified by Toppr Upvote 52. The purpose of the practical is for students to subject the nails to differing conditions and after time look at how much rust appears on each nail. So the presence of air and water vapour in air are two necessary conditions for rusting of iron.
The reaction of the rusting of iron involves an increase in the oxidation state of iron accompanied by a loss of electrons. 1In the first test tube containing iron nail we put some anhydrous calcium chloride and closed its mouth with a tight cork. In this experiment youll discover what kind of conditions help rust form or prevent it.
The reactants of this chemical reaction are iron water and oxygen and the product is hydrated iron oxide better known as rust. - Electrochemical oxidation of iron with oxygen is called rusting. This is subsequently oxidized to Fe 3 by exposure to oxygen.

.png)