Stunning Sodium Hydroxide And Hcl Balanced Equation

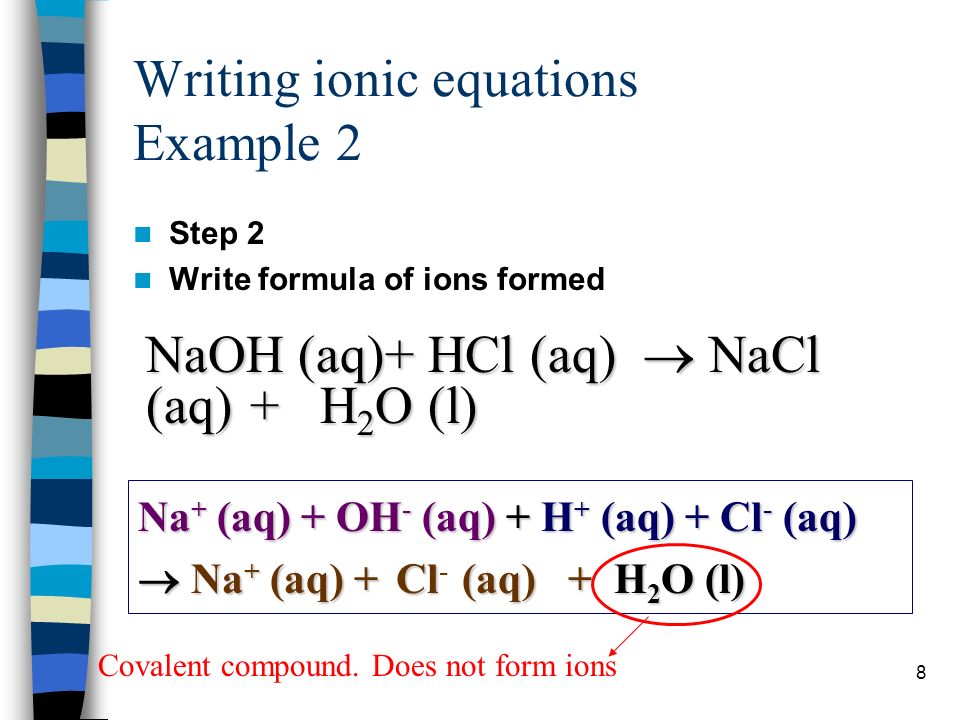
This means that we will split them apart in the net ionic equation.
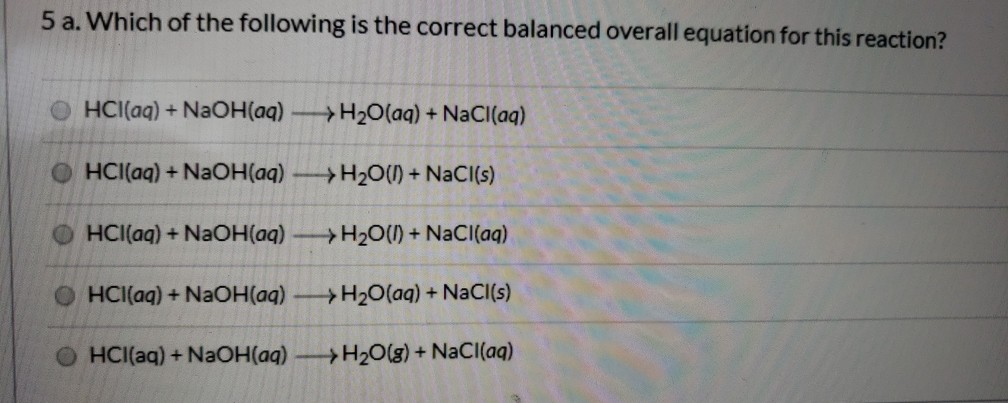
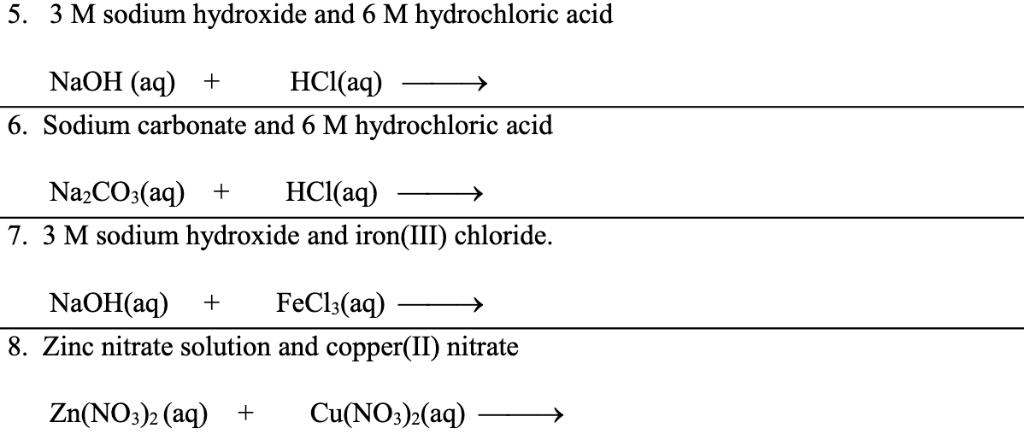
Sodium hydroxide and hcl balanced equation. Q3 Write a balanced equation for each of the following reactions. Use this information and the equation to answer any questions that follow. If Algae will become a difficulty the pool could have for being drained and washed having a 5050 mixture of muriatic acid and h2o.
A total of 872 mimiters mL 042 NaOH reacts with 218 of HCl of unknown concentration. Sodium hydroxide diluted solution. This reaction takes place in a nitrogen atmosphere.
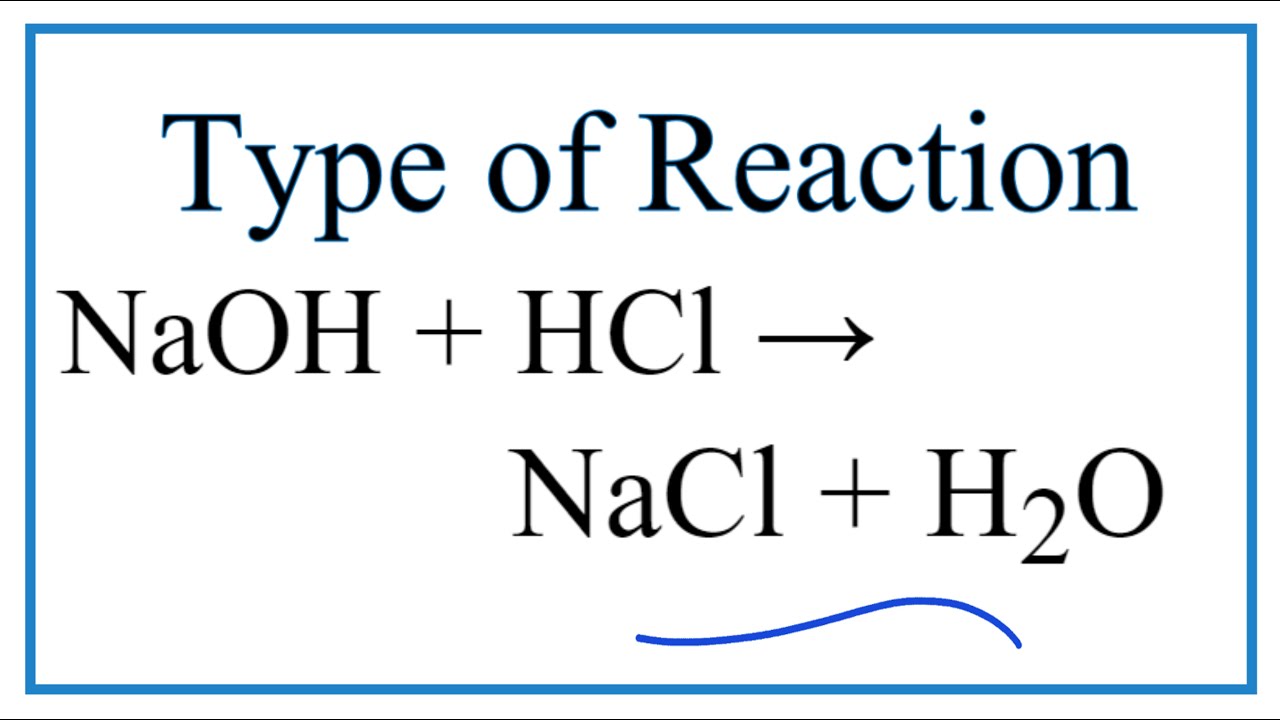
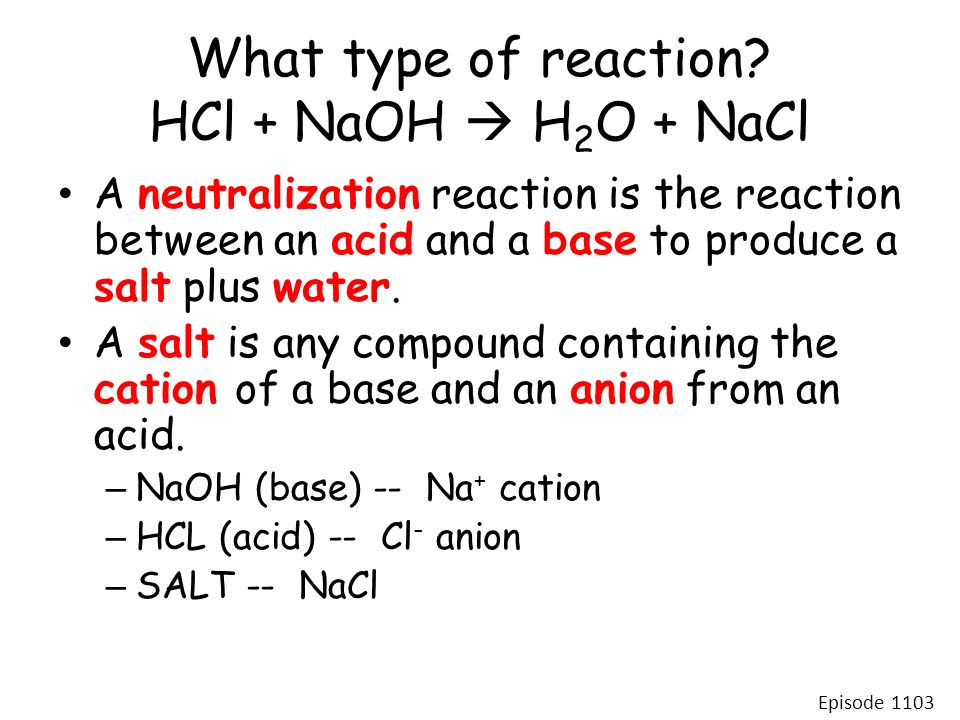
Hydrochloric acid HCl reacts with Sodium Hydroxide NaOH to form a colourless aqueous solution of Sodium Chloride NaCl salt. Lets see how a neutralization reaction produces both water and a salt using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide. Write the balanced chemical equation representing the reaction of hydrochloric acid and sodium hydroxide.
Type of reaction double replacement E Sodium Carbonate and HCl Reactants 1. HCl NaOH NaCl H₂O Hydrochloric Acid and Sodium Hydroxide Balanced Equation. Hydrochloric acid HCl reacts with Sodium Hydroxide NaOH to form a colourless aqueous solution of Sodium Chloride NaCl salt.
So the equation should be balanced and in this case it is already balanced. Lets see how a neutralization reaction produces both water and a salt using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide. How to Balance the Net Ionic Equation for HClO 4 NaOH The reaction of Perchloric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base.
Write the correct formulas of. HCl NaOH NaCl H2O Now as per the law of conservation of mass mass is neither created nor destroyed in a chemical reaction. Formula Equation For Sodium Hydroxide With Hydrochloric Acid.