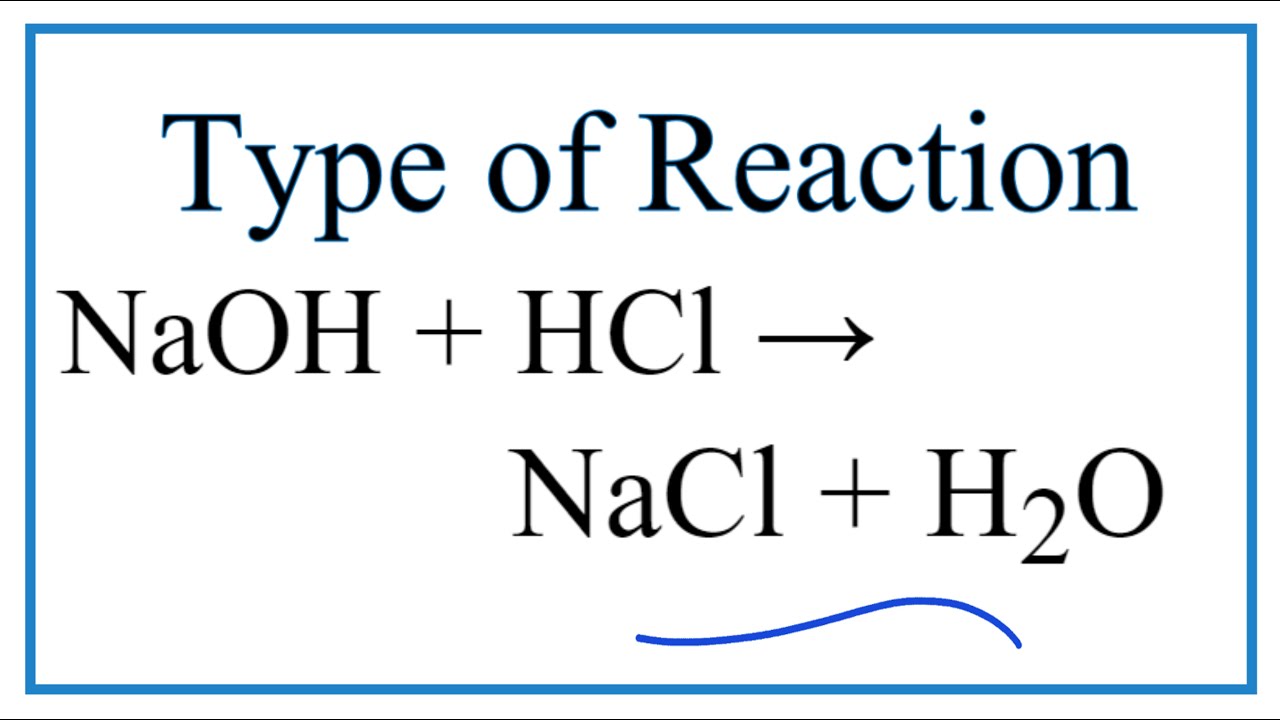
Best Hcl Plus Naoh Balanced Equation

What is the limiting reactant in this chemical equationIn the neutralization of 10 M HCl and 10 M NaOH NaOH aq HCl aq - NaCl aq H2O.
Hcl plus naoh balanced equation. 2HCl 2NaOH 2NaCl H2O 2 H C l 2 N a O H 2 N a C l H 2 O. K 4 FeCN 6 H 2 SO 4 H 2 O K 2 SO 4 FeSO 4 NH 4. Hydrochloric acidHClreacts with Sodium HydroxideNaOHto form a colourless aqueous solution of SodiumChloride NaCl salt.
But since sodium is always soluble in water at temperatures below the boiling point of. The heat exchanged by the reaction qreaction can be used to determine the change in enthalpy of the reaction. Chemical Balanced Equation In hypochlorite ion chlorine atom is in the 1 oxidation state.
Fe Au Co Br C O N F. HCl aq NaOH aq NaCl aq H2O l heat Calculate the number of moles of base you add to determine the molar heat of neutralization expressed using the equation ΔH Q n where n is the number of moles. Formula Equation For Sodium Hydroxide With Hydrochloric Acid.
Examples of complete chemical equations to balance. The balanced chemical equation representing the neutralization of hydrochloric acid with sodium hydroxide is. So the equation should be balanced and in this case it is already balanced.
Sodium Hydroxide Hydrochloric Acid Balanced Molecular Equation Complete And Net Ionic Equation. Hydrochloric Acid And Sodium Hydroxide Balanced Equation The pH scale actions a substances acidity but an acid can even more be classified as both a weak acid or even a robust acid. KMnO 4 HCl KCl MnCl 2 H 2 O Cl 2.
Hydrochloric acid HCl reacts with Sodium Hydroxide NaOH to form a colourless aqueous solution of Sodium Chloride NaCl salt. The balanced chemical equation representing the neutralization of hydrochloric acid with sodium hydroxide is. The limiting reagent row will be highlighted in pink.