Amazing Examples Of Unbalanced Equations
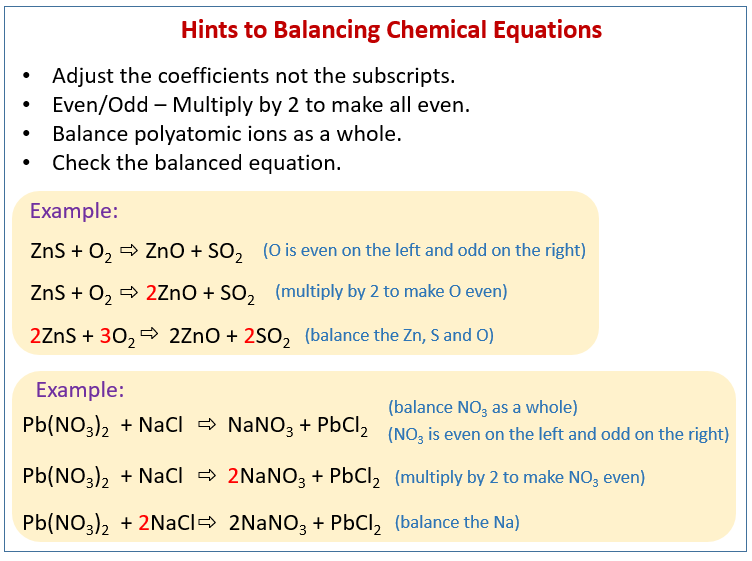
Examples of using the twos and threes method to balance chemical equations even odd technique examples and step by step solutions Chemistry Balancing chemical equations Atoms can neither be destroyed nor created during a simple chemical reaction.
Examples of unbalanced equations. Easy Equation Balancing Examples. In this example it can be difficult to know where best to start as 3 of the elements appear once in the reactants and products. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button.
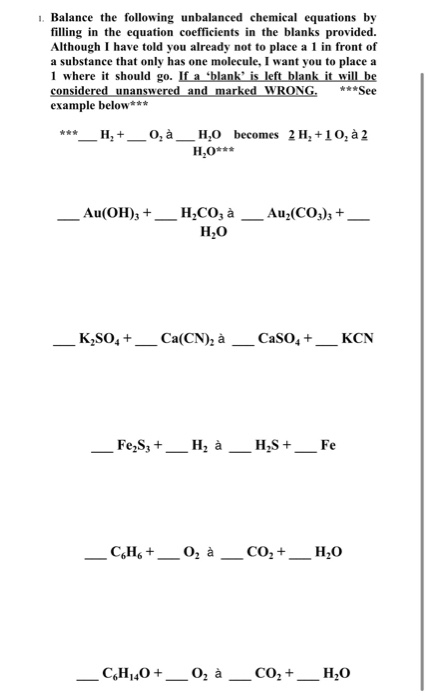
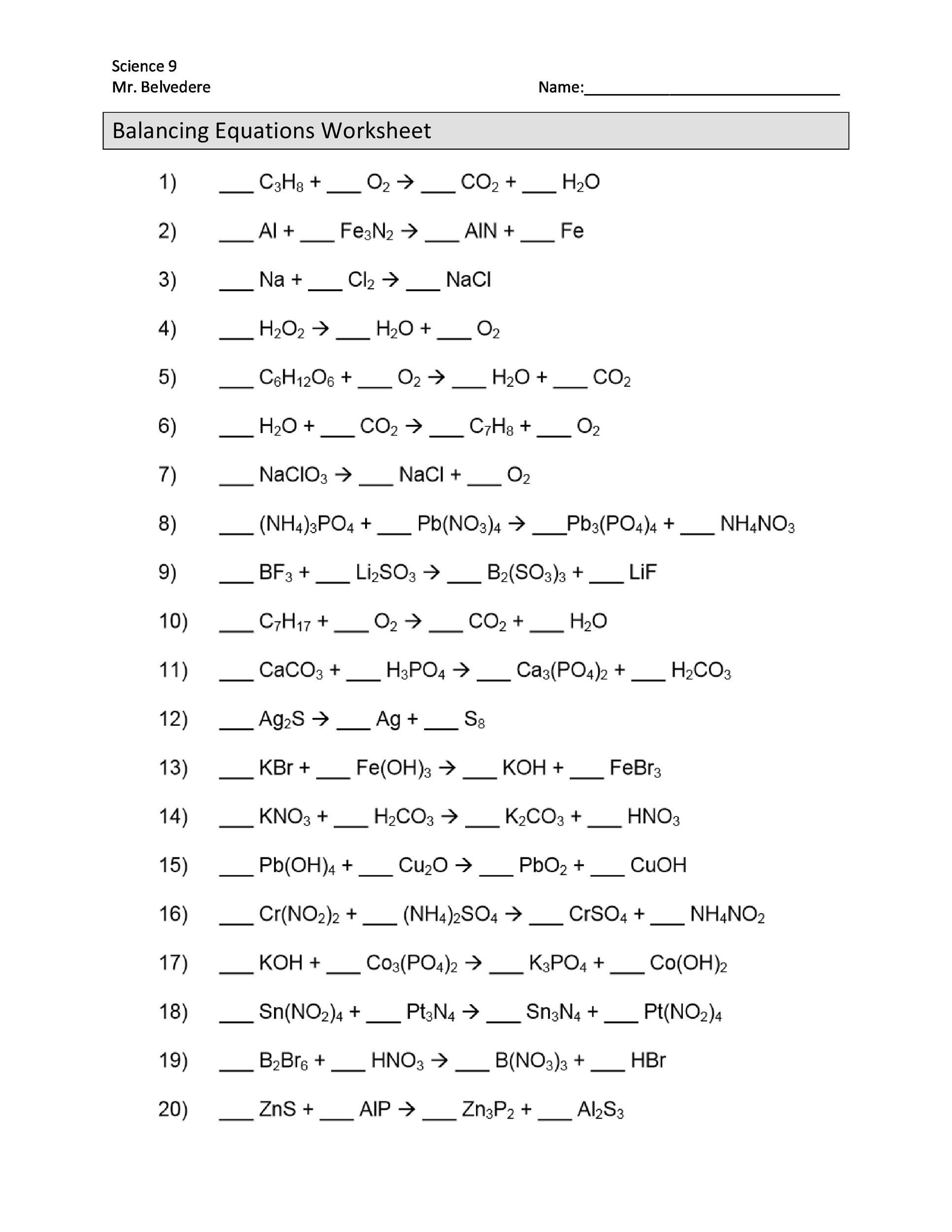
The word equations for a few of these reactions have been provided though most likely youll be asked to provide only the standard chemical equations. Therefore this is an unbalanced chemical equation. Balancing Equations Difficult Ones In AQA exams there are commonly some quite difficult balancing equations questions.
2Fe 2 O 3 3C 4Fe 3CO 2. Balanced or easy-to-balance chemical equations will be seen in the following examples. Ionic charges are not yet supported and will be ignored.
Now if you notice the element Fe has the subscript 2 beside itself signifying the number of atoms. How do you balance chemical equations examples. Examples of Balancing Chemical Equations.
The next most obvious unbalanced part is that there are now three NH4groups on the right but only one on the left hand side. For example this equation for the reaction between iron oxide and carbon to form iron and carbon dioxide is unbalanced with respect to mass. Fe Au Co Br C O N F.
What is the example of unbalanced chemical equation. FeCl3 3NH4OH --- FeOH3 3NH4Cl And if you count up the atoms on each side you will see that this is now. Note that if you have 1 of something it does not get a coefficient or subscript.