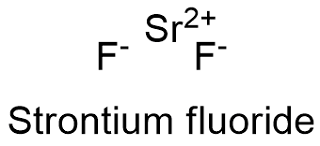Heartwarming Strontium Fluoride Balanced Equation

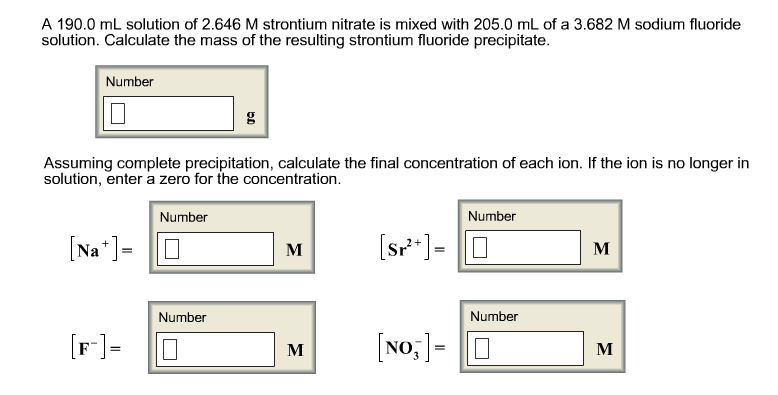
The mass of the resulting strontium fluoride precipitate is 378 g.
Strontium fluoride balanced equation. You must KNOW the formulas and charges of the polyatomic ions. 7Nas Fg Na7Fs Nas Fg NaFs Nas F2g NaF2s. BaOH2BaOH2 is a strong electrolyte.
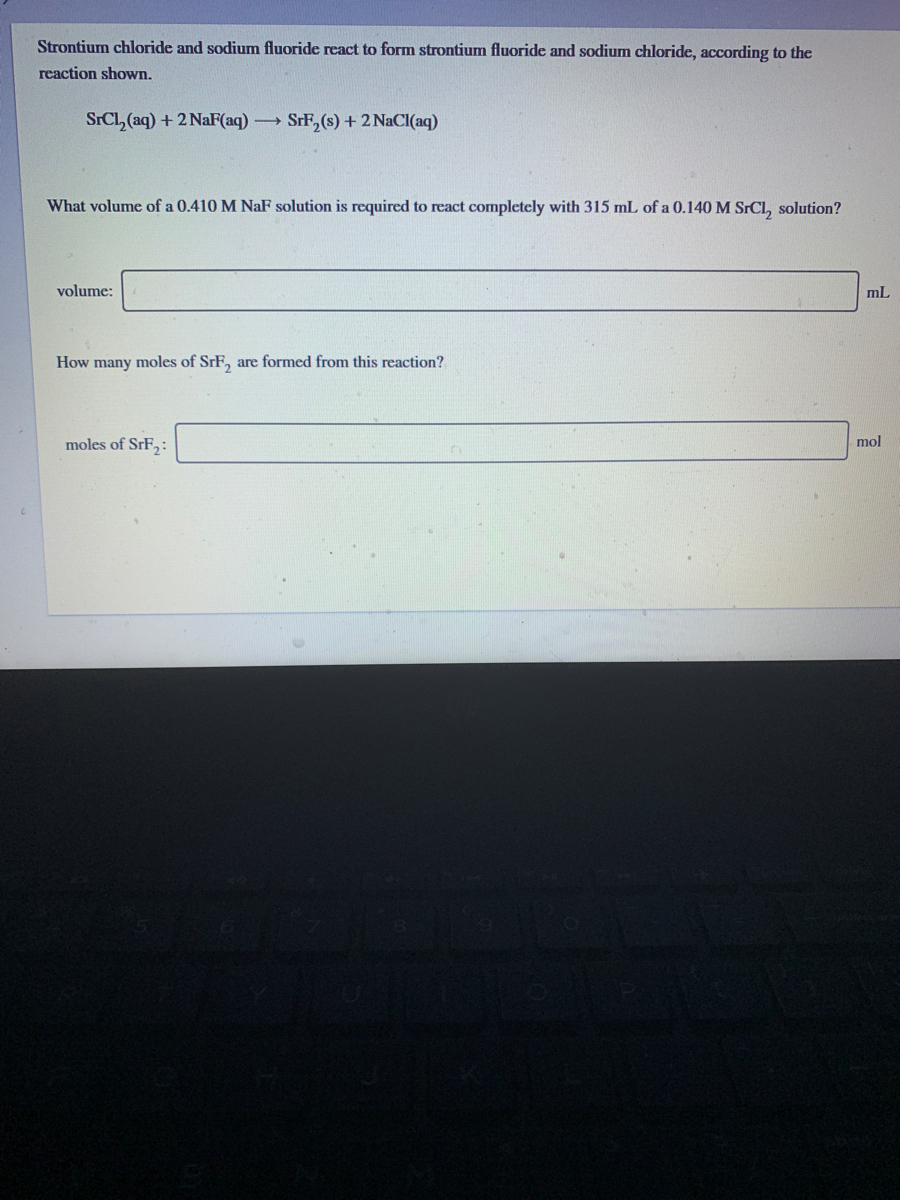
D A 500-milliliter sample of 300 x 10-3 molar FeSO 4 solution is added to 500 milliliters of 400 x 10-6 molar NaOH. SrCl2aq2NaFaq SrF2s2NaClaq What volume of a 0650 M NaF0650 M NaF. FeF2s Fe2aq 2 Faq A sample of iron fluoride FeF2 is dissolved in water and a saturated solution is obtained.
The half-life for beta decay of strontium. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. Fe Au Co Br C O N F.
The product is what would be expected in a synthesis. B Write the expression for the solubility product constant Ksp and calculate its value. Strontium fluoride SrF2 Strontiumfluoride.
Strontium Chloride Sodium Fluoride Produces Strontium Fluoride Sodium Chloride. This is the lowest possible ratio. The balanced equation will appear above.
Check Your Solution The overall charge on the compound strontium fluoride is zero and the equation is balanced. Srs F 2g. Use symbols when appropriate.