Fine Beautiful Water Softening Ppt

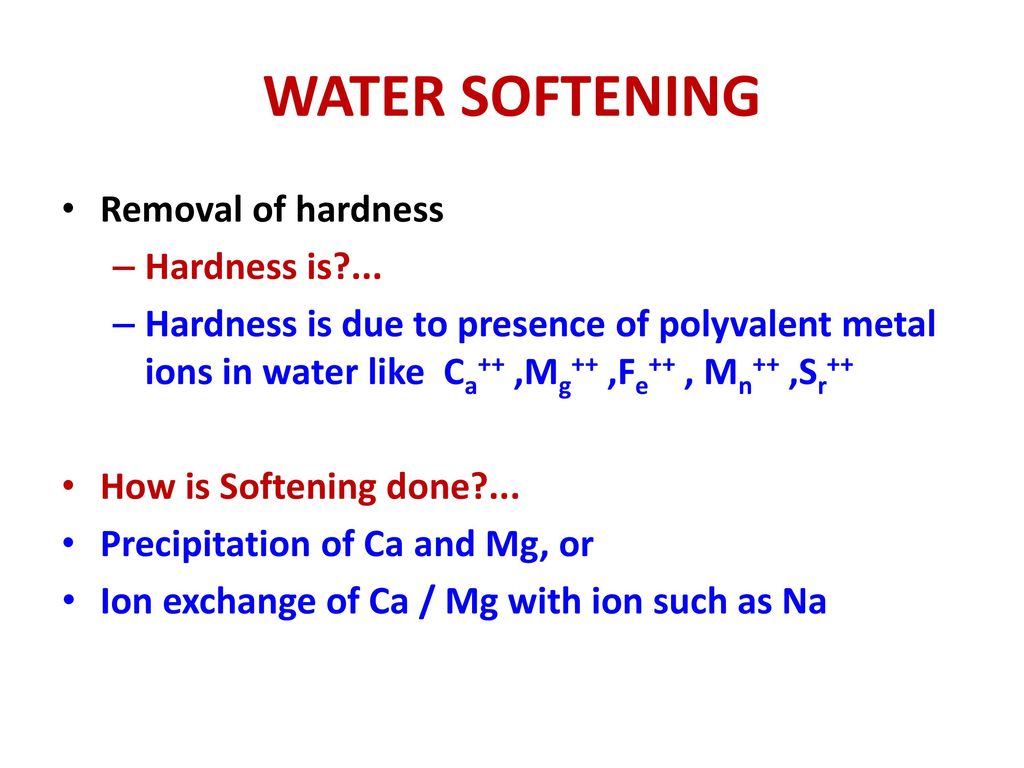
1 graingallon 171 mgL CaCO 3 Total hardness is a test of overall water quality.
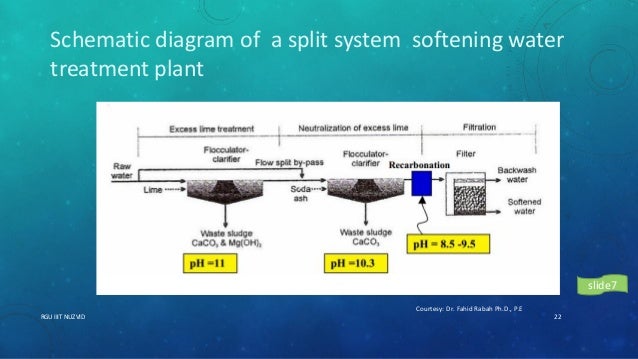
Water softening ppt. Lime-Soda Water Softening Process. For CE 3234 Last modified by. Contaminants removed by the water softening ion.
12201996 50414 PM Document presentation format. Softeners may also remove as much as 5-10 ppm parts per million. Water softening and hardness removal.
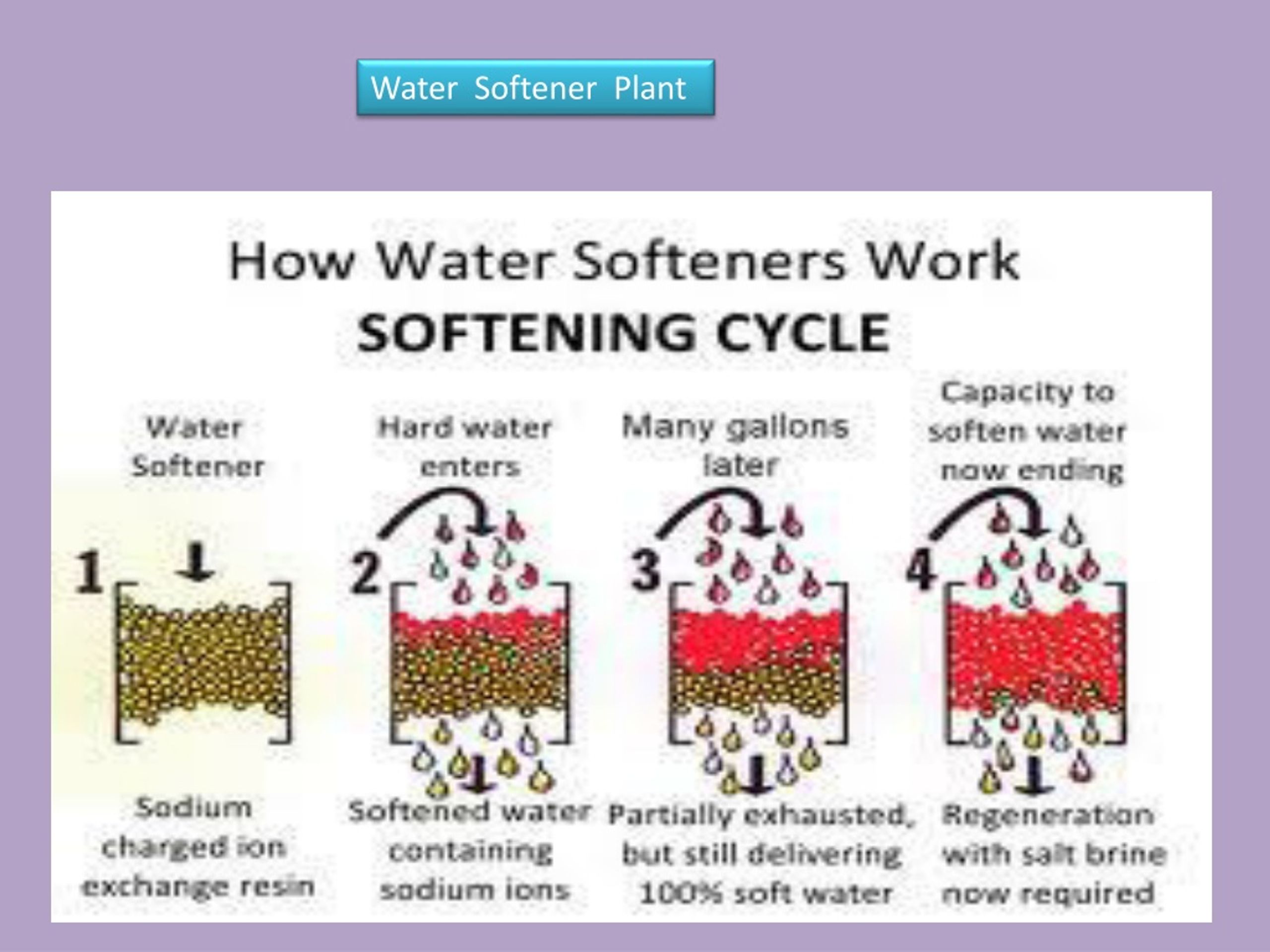
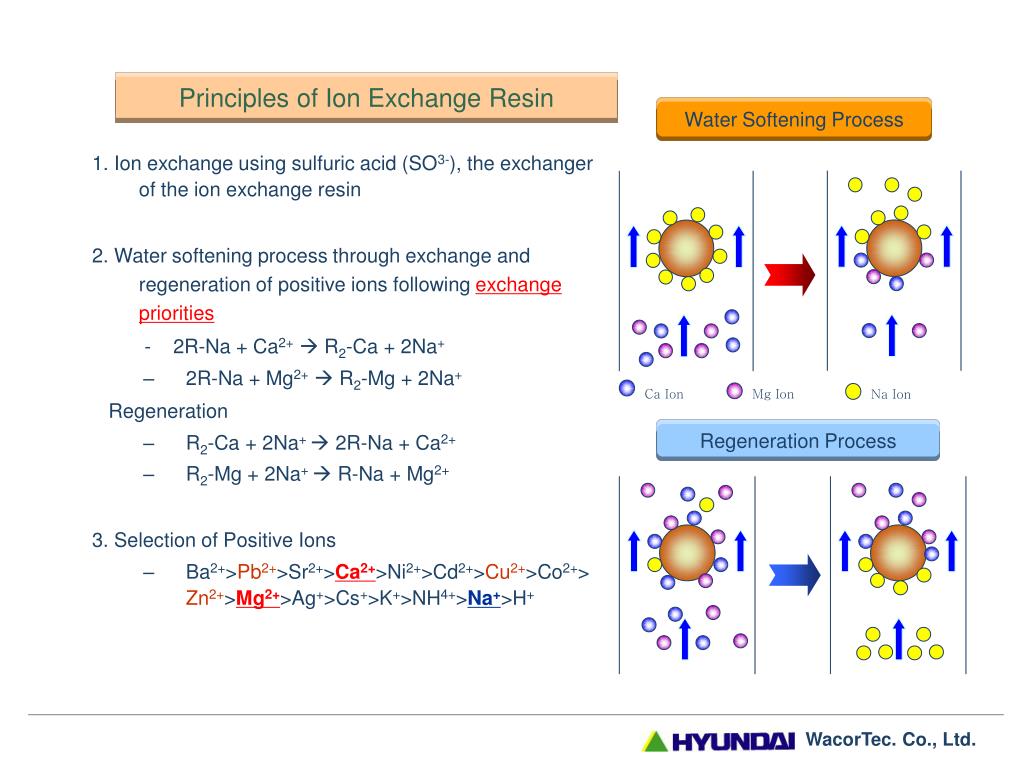
In the ion exchange process sodium ions are used to coat an exchange medium in the softener. Ppm is equal to milligrams per liter or mgL of iron and manganese. It flows through the resins and is carried to drain with addiction of calcium and magnesium released from resins.
Water softeners usually use sodium Na as the exchange ion. Zeolite process for water softening has become a commercial success for the reason that zeolite can be easily regenerated. The softeners are the equipment that allow to make the softening of the water by ion exchange process.
Basics of Water Softening by Ion Exchange Author. PowerPoint PPT presentation free to view. This process is now obsolete but was very useful for the treatment of large volumes of hard water.
Water used in recharging a water softener may overload or reduce the effectiveness of small septic or sewer systems. Values near 150 mgL are generally ideal from an aesthetic viewpoint. 5232012 54602 PM.