Outstanding How To Write Incomplete Combustion Equations

For example here is one possible equation for the incomplete.
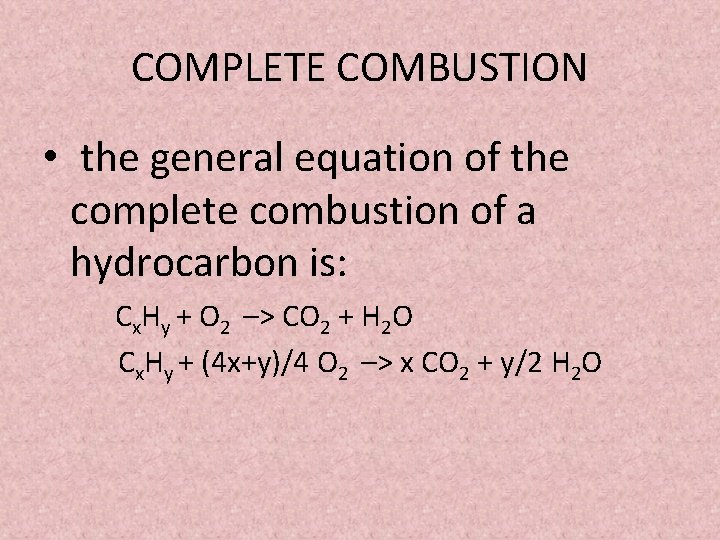
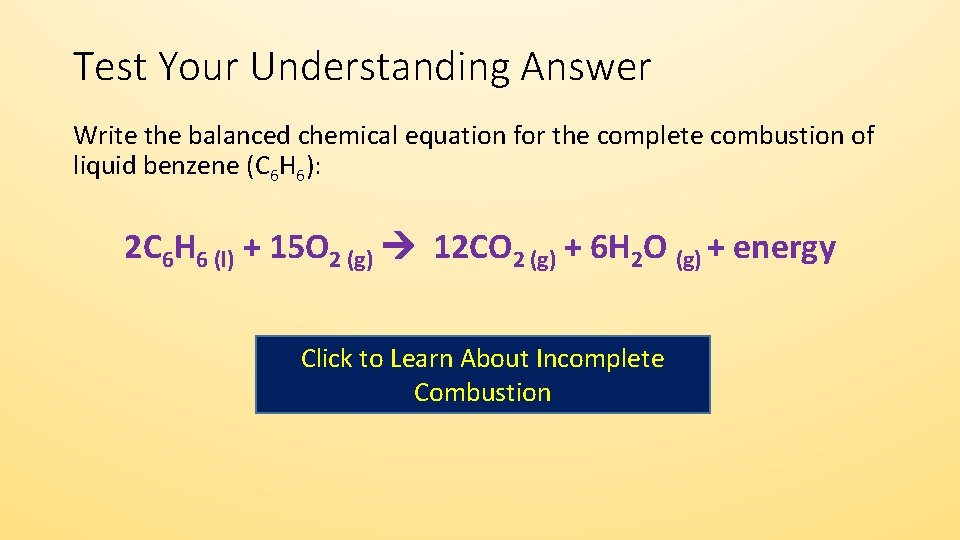
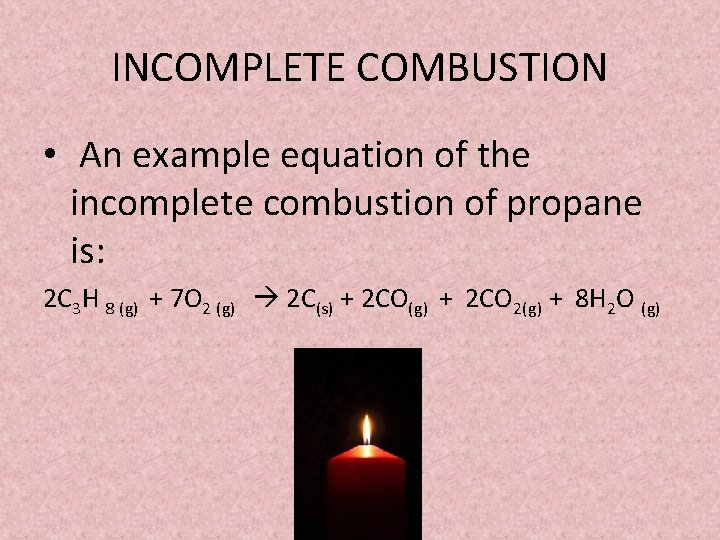
How to write incomplete combustion equations. This is mostly thermal energy but. A Incomplete Combustion Equation - Multiple carbon products Hydrocarbon Oxygen Carbon Carbon monoxide water When writing equations with incomplete combustion it is advisable to include only one carbon product otherwise there will be multiple solutions to the equation. Water is still produced but carbon monoxide and carbon are produced instead of carbon dioxide.
Complete combustion does NOT give carbon monoxide or soot. There oxygen combines with another molecule to produce. It is quite important that you can write properly balanced equations for these reactions because they often come up as a part of thermochemistry calculations.
It is an example of an exothermic reaction a reaction that releases energy to the surroundings. Incomplete combustion i. C 3 H 8 O 2-- H 2 O CO 2.
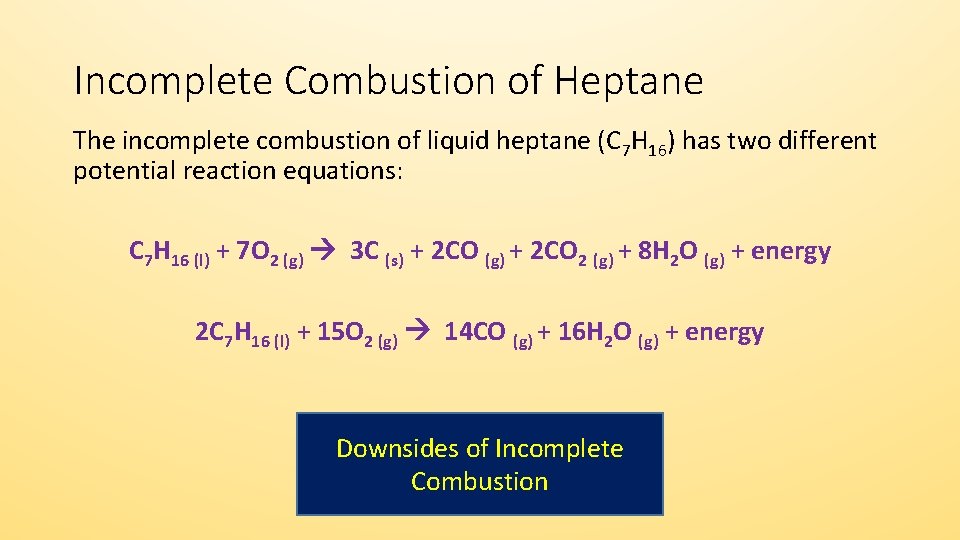
C 3 H 8 5O 2-- 4H 2. TagsAcid-base reaction theories Alchemy Analytical chemistry Astrochemistry Biochemistry Crystallography Environmental chemistry Food chemistry Geochemistr. Before we balance the incomplete combustion of pentane lets remember first that the balanced equation for the complete combustion for pentane will be written as follows.
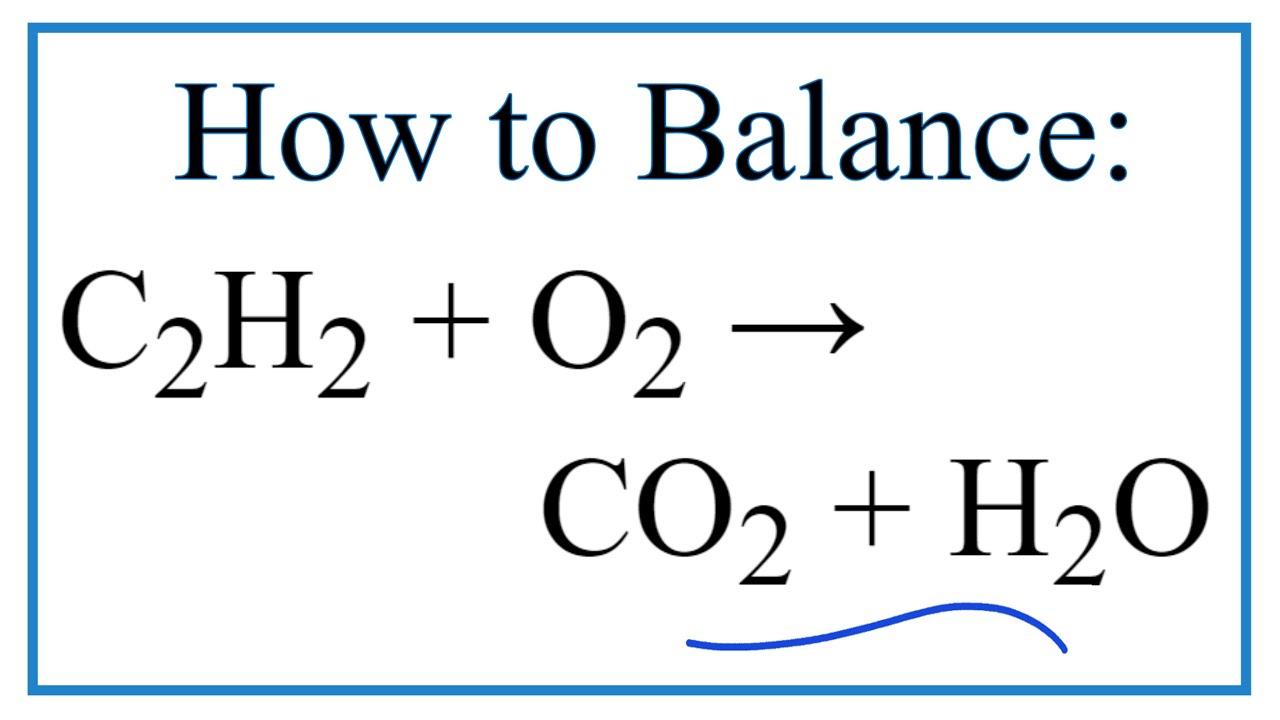
C9H 20l 27 2 O2g 8CO2g COg 10H 2Ol. Acetylene aka ethyne C2H2 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. The key difference between complete and incomplete combustion is that complete combustion takes place when there is a constant and enough oxygen supply whereas incomplete combustion takes place when there isnt enough oxygen supply.
808 Explain why the incomplete combustion of hydrocarbons can produce carbon and carbon monoxide. All right now we have another combustion reaction instead of instead of ethylene we now have ethane c2h6 has two carbons and six hydrogen atoms in each molecule of ethane and it is reacting its ethane gas its reacting with molecular oxygen in gaseous form and they combust to form carbon dioxide gas and liquid water and like weve seen in previous examples this chemical equation is not. Write the formula for the given reactant.