Ideal Chemical Reaction Formula

4M3Q 2 2M 2 O 3.
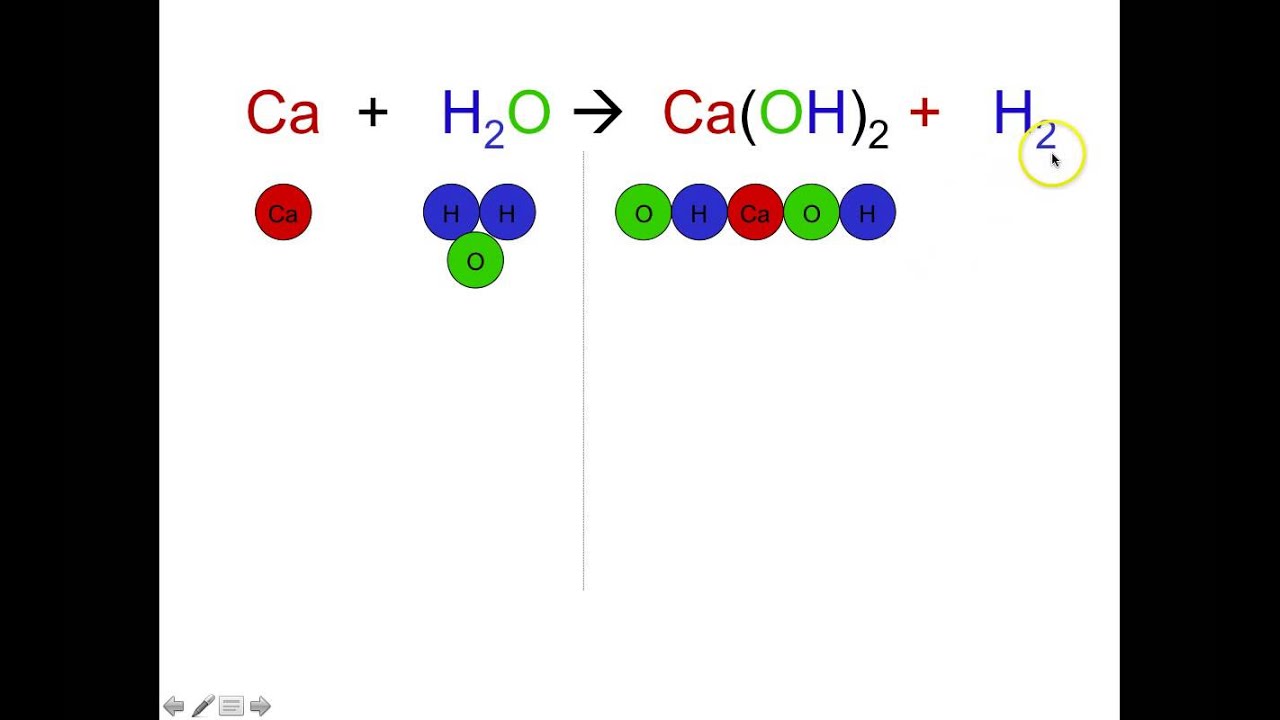
Chemical reaction formula. What come out of a chemical reaction. For example iron Fe and sulfur S combine to form iron sulfide FeS. Generally we represent it with a chemical equation in which the reactants substances that are broken apart are written on the left and the products a new substance that form are written on the right.
Dealing with the quantitative aspect of chemical reactions is called reaction stoichiometry. By using this website you agree to our Cookie Policy. Draft a skeleton equation wherein reactants formula is on the left and products o right side of the equation.
The molecules of a substance are broken apart and rearranged into new substances. A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side. The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas liquid water sodium ions and acetate ions.
The substances used in the beginning of a chemical reaction are called the reactants usually found on the left side of a chemical equation and the substances found at the end of the reaction are known as the products usually found on the right side of a chemical equation. They could draw a picture of the chemical reaction. Free Chemical Reactions calculator - Calculate chemical reactions step-by-step This website uses cookies to ensure you get the best experience.
CH 4 2O 2 CO 2 2H 2 O. Chemical reactions can be represented on paper with the help of chemical equations an example for which is represented below for the reaction between hydrogen gas and oxygen gas to form water. Chemical reactions can be written in chemical equation form which should always be balanced.
Chemical Reaction Formula Examples. The arrow signifies that the reaction forms or yields iron sulfide the product. They consist of chemicalor structural formulasof the reactants on the left and those of the products on the right.