Stunning Ethane Burns In Oxygen Balanced Equation

Complete combustion does NOT give carbon monoxide or sootCheck me out.
Ethane burns in oxygen balanced equation. 17 Riverdale Avenue Yonkers N Y 10701 Phone. C What mass of O 2 in grams is required for complete combustion of 136 of ethane. What happens when ethane reacts with oxygen.
What type of reaction is propane and oxygen. Ethane is an alkane with the chemical formula C2H6. What is the balanced equation for ethane.
B Write the balanced equation for the reaction. Get the detailed answer. D What is the total mass of products expected from the combustion of 136 g of ethane.
Ethane C 2 H 6 burns in oxygen. The balanced equation for the combustion of butane combines two molecules of butane with 13 oxygen molecules. Ethane C_2H_6 reacts with molecular oxygen to produce carbon dioxide and water.
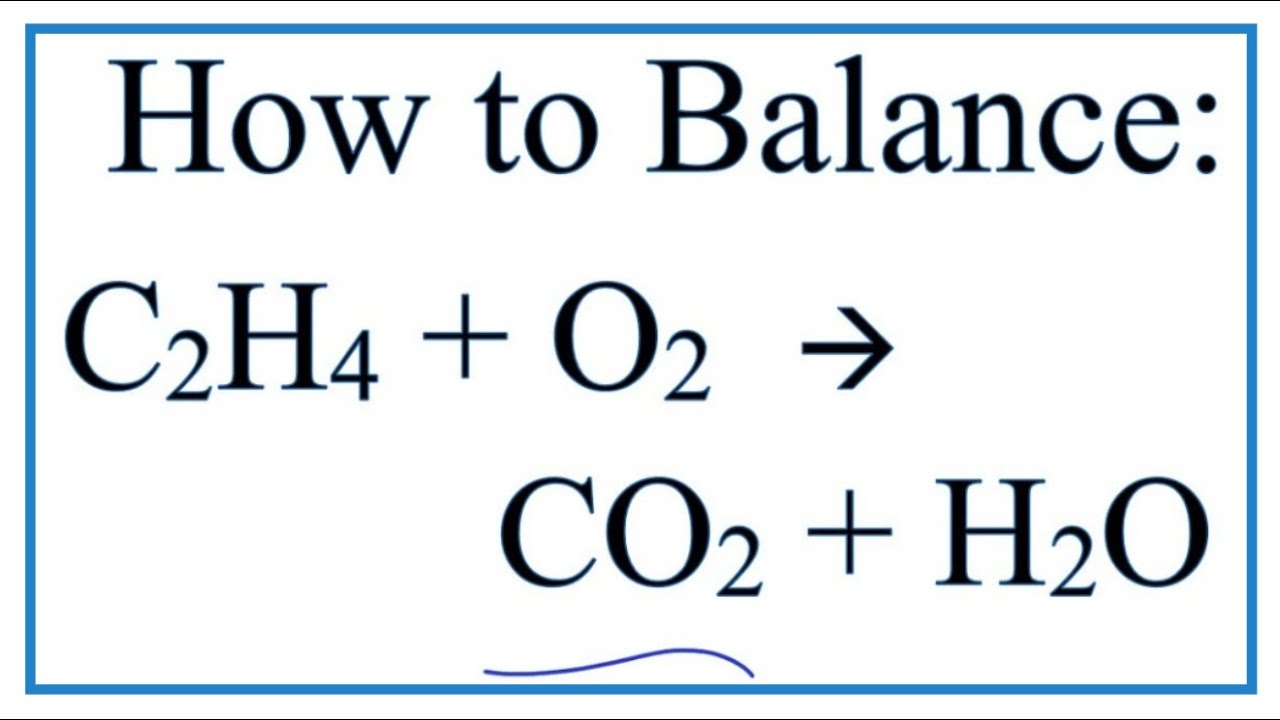
The equation for the combustion of ethane is 2C2H67O24CO26H2O 2 C 2 H 6 7 O 2 4 C O 2 6 H 2 O. Ethane C2H6 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. C2H2 O2 2 CO2 H2O need 25 O2s to balance the Os Ethane burn in the presence of oxygen to form carbon dioxide and water and release of energy in the form of heat and light.
Balance the equation and calculate enthalpy change eqDelta H Correct answer to the question. Ethane C2H6 burns in oxygen. If not enough oxygen is present for complete.