Awesome Koh In Water Equation
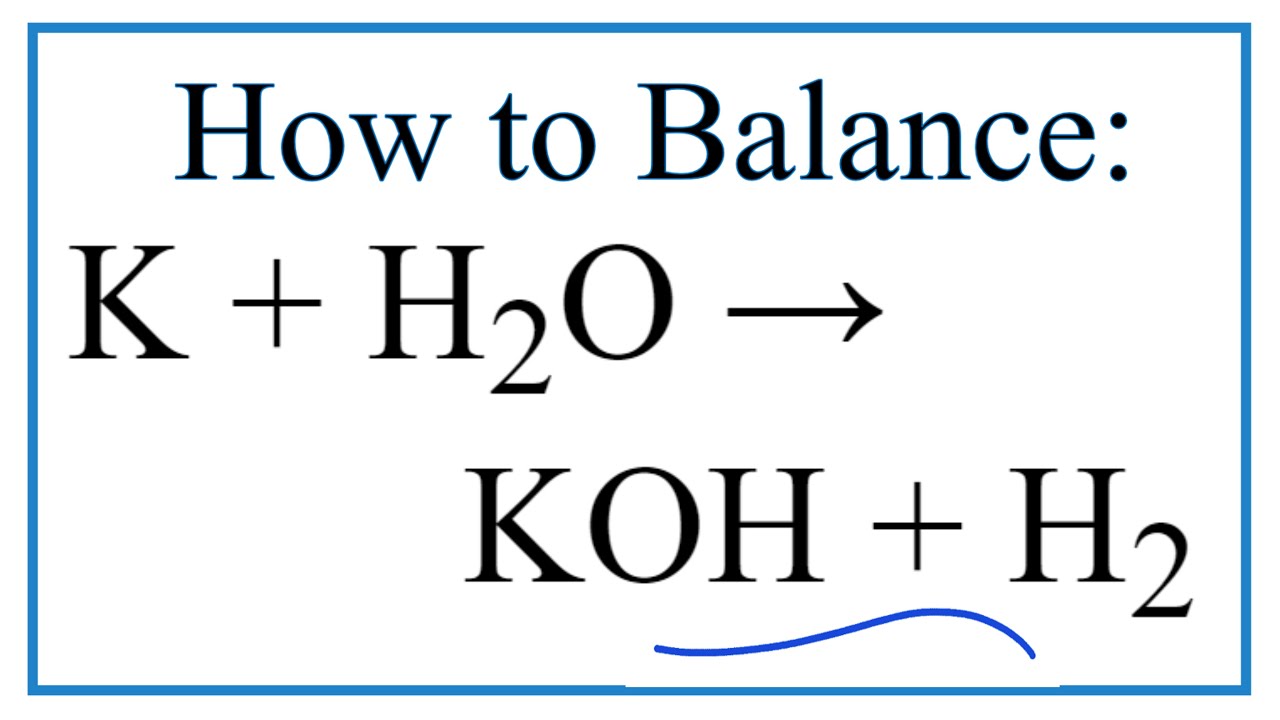
Potassium hydroxide is an inorganic compound with the formula K OH and is commonly called caustic potash.
Koh in water equation. In water these bases dissociate into metal cation and OH - ion. Due to complete dissociation initial concentration of KOH is equal to OH-concentration. Strong bases dissociate completely in water.
Therefore OH - 005 M. What happens when you mix KOH and HNO3. Although the pH of KOH or potassium hydroxide is extremely high typical solutions typically range from 10 to 13 the exact value depends on the concentration of this strong base in water.
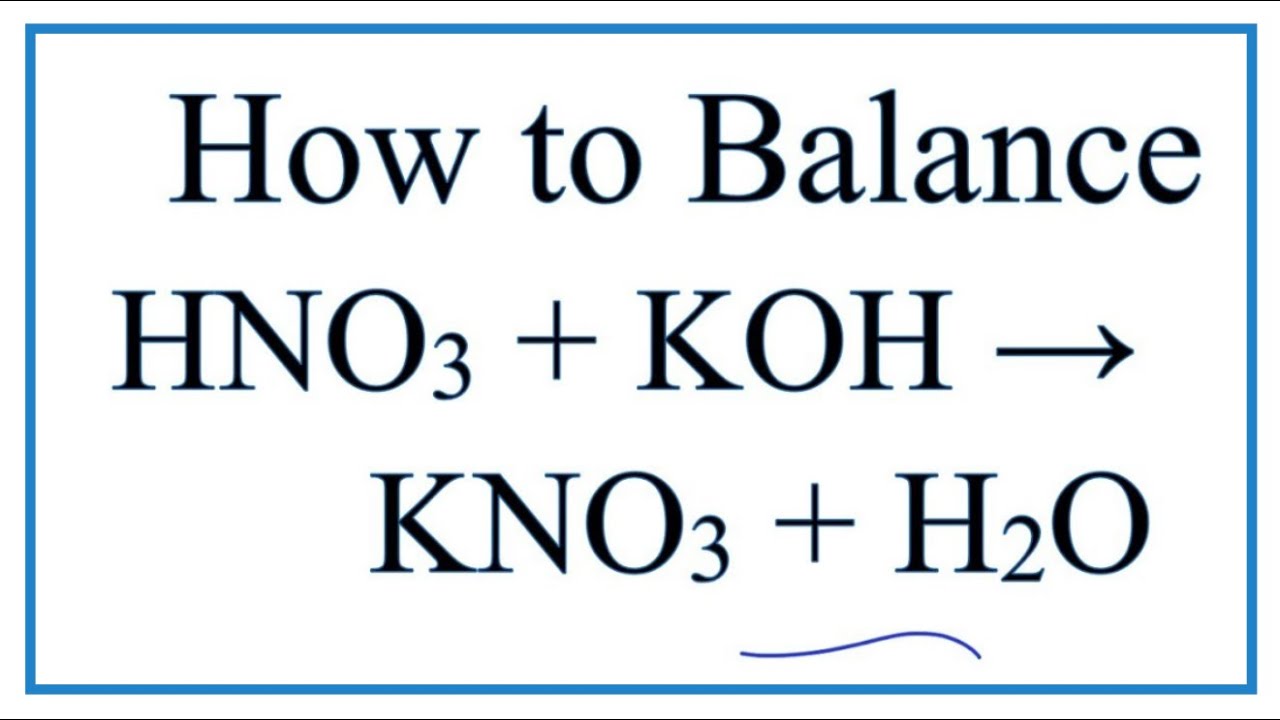
HNO3 KOH H2O KNO3 Neutralization produces a salt and water. Net ionic equation for hcl and koh This is a neutralisation reaction - KOH is potassium hydroxide and HF is hydrofluoric acid. KOHHClKClH2O is a neutrailization as H ions combines with OH ions to form water.
For example if your KOH is 100 pure hydroxide then when mixed with water it ionizes to give potassium ions K and OH- ions. The potassium has a charge of K and hydroxide has a charge of OH. Some of strong bases are NaOH KOH Ba OH 2.
To determine the precise concentration of a KOH solution you must titrate it with another standard solution such as KHP. You will use the KHP standard solution that you made in Part 1 in a series of. Along with sodium hydroxide NaOH KOH is a prototypical strong baseIt has many industrial and niche applications most of which exploit its caustic nature and its reactivity toward acidsAn estimated 700000 to 800000 tonnes were produced in 2005.
Homework Statement The net ionic equation for the reaction between aqueous solutions of HF and KOH is___. Relationship of pH pOH 14. This is because KOH is not available in very pure formit absorbs water from the air.