Favorite Incomplete Combustion Formula

The general formula for incomplete combustion is shown below.
Incomplete combustion formula. 4CH4 5O2 2CO 8H2O 2C. Improving primary secondary and tertiary air distribution this includes verification when a boiler is commissioned or after major modifications. If not enough oxygen is present for complete combustion incomplete combustion occurs.
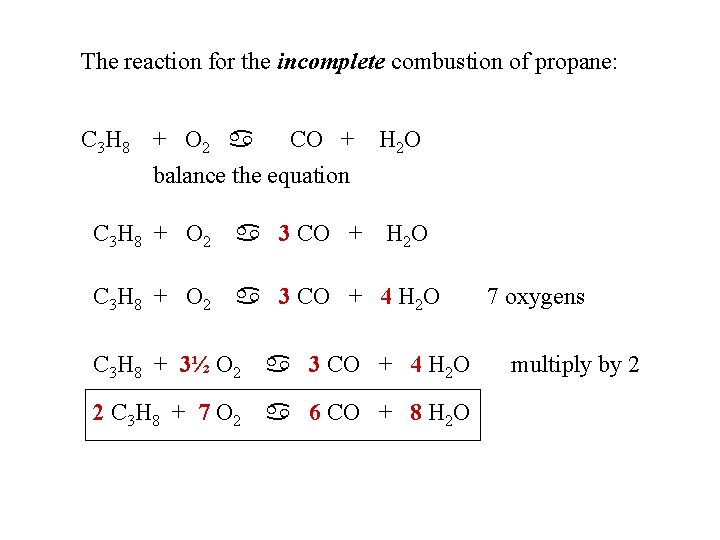
As the amount of oxygen is decreased from a ratio of 2 to 15 to 1 in the above three equations the combustion products change from carbon dioxide to carbon monoxide to carbon or soot. Recognizing the characteristics and balancing incomplete combustion reactions. 2 C3H8 9 O2 4 CO2 2 CO 8 H2O Heat.
Thus significant improvement can be obtained in new but also in existing boilers by. Combustion of a Renewable and Fossil Fuel. Methane oxygen carbon and water.
Methanol water a There are several reasons why the increase in temperature is less than expected. In many cases these substances can be toxic. Lets start with what complete combustion is.
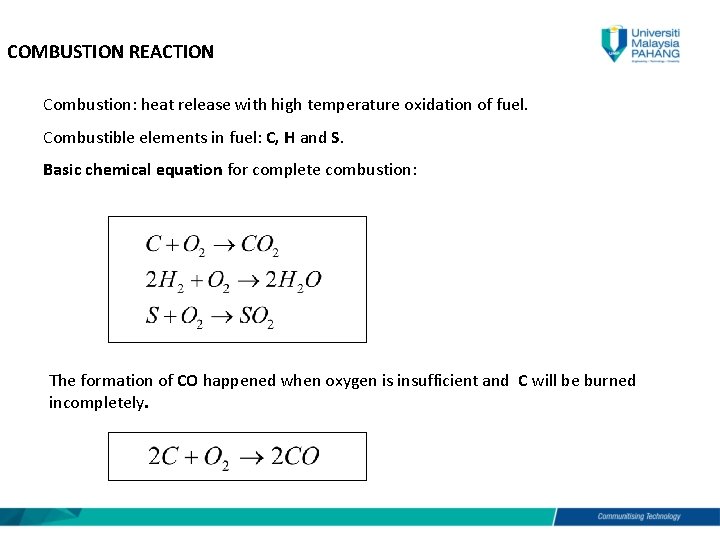
Chemistry The Mole Concept Determining Formula. Complete combustion is the textbook equations for mixing hydrocarbons with oxygen burning all of it and reducing to CO2 and water. Incomplete combustion of methane producing soot Burns with a yellow flame.
The Combustion of Hydrocarbons - Chemistry. Hydrocarbon Oxygen Carbon monoxide Carbon Water The byproducts may vary according to the amount of oxygen that is involved in the combustion. Could it happen the reaction should be described by the equation C2H6 2 O2 CO C 3 H2O From a purely stoichiometric standpoint it is to be pointed out that in the absence of the arbitrary constraint concerning the equal amounts of C.