Glory Ethane Complete Combustion Equation
Ethane which is a saturated hydrocarbon undergoes complete combustion to give carbon dioxide and water as the products.
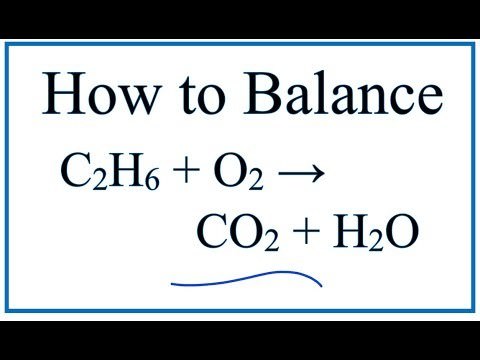
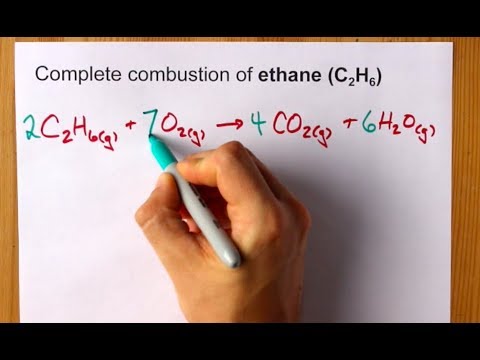
Ethane complete combustion equation. This balanced equation shows the combustion of ethane. Write and balance the equation for the complete combustion of ethane C2H6. The Complete Combustion of Ethane.
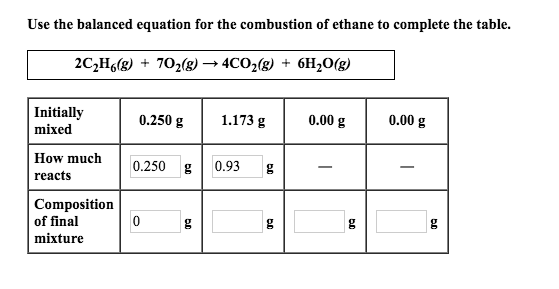
Lets compose the reaction equation. In order to balance C2H6 O2 CO2 H2O youll need to watch out for two things. Ethane oxygen carbon dioxide water energy 2C 2 H 6 g 7O 2g 4CO 2g 6H 2 O l The reaction is exothermic it gives out heat.
Write and balance the equation for the complete combustion of ethane C2H6. The balance equation for the complete combustion of ethane. Fuel O2 CO2 H2O You would then balance the chemical equation.
Click hereto get an answer to your question The balance equation for the complete combustion of ethane is. It takes place when there is a good supply of oxygen. The reaction also has a negative enthalpy change ΔH value.
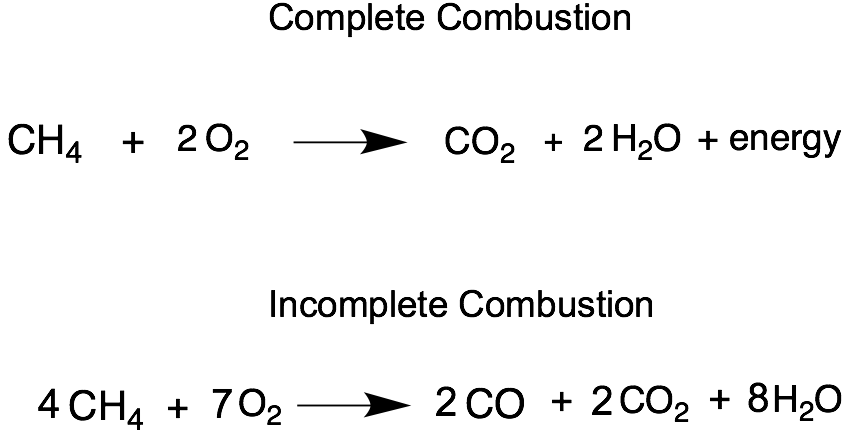
Calculates the ratio of waterdry gas in the stack gas for both complete and partial combustion of ethane. The products are the same carbon dioxide and water. First be sure to count all of C H and O atoms on each side of the.
The balanced chemical equation for the complete combustion of ethane is. 4CO2 6H2O An equation for the incomplete combustion of butane in oxygen is A major problem associated with the incomplete combustion of soft coal is pollution. Acetylene aka ethyne C2H2 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O.